AF is the most common sustained cardiac dysrhythmia, affecting 2–3% of the population.1 AF is associated with significant impairments in quality of life, as well as significantly increased risk of adverse cardiovascular outcomes (e.g. heart failure, thromboembolism and premature mortality). As such, AF management is focused on improving arrhythmia-related symptoms as well as reducing morbidity associated with AF by using strategies to meaningfully reduce AF-associated healthcare usage.2
While previous studies suggested that control of the ventricular rate was comparable to a strategy of sinus rhythm maintenance in patients with established AF, the recent EAST-AFNET 4 randomised trial demonstrated that early rhythm control (i.e. within the first year after diagnosis) provided significant benefit.3 Specifically, early rhythm control was associated with significant reductions in the composite primary outcome of cardiovascular death, stroke and hospitalisation for worsening heart failure and acute coronary syndrome by 21% (from 5.0% per year to 3.9% per year) over a median follow-up of 5.1 years, which was driven by a significant reduction in cardiovascular death (1.0 versus 1.3% per year; HR 0.72; 95% CI [0.52–0.98]) as well as the incidence of stroke (0.6 versus 0.9% per year; HR 0.65; 95% CI [0.44–0.97]).4 Consistent with major guidelines, rhythm control in the EAST-AFNET 4 trial was predominantly pharmacological, with the majority of patients receiving class Ic antiarrhythmic drug therapy.5
While antiarrhythmic drug therapy is relatively superior to placebo in the prevention of arrhythmia recurrence, these agents have only modest efficacy in maintaining sinus rhythm.6,7 Moreover, antiarrhythmic drug therapy is associated with significant side effects, including, in the case of sotalol, an increase in all-cause mortality (RR 2.23 versus placebo; 95% CI [1.03–4.81]).8
This may explain the apparent lack of benefit observed with pharmacological rhythm control in previous studies. Highlighting this point was a time-dependent on-treatment analysis of the landmark AFFIRM trial, which demonstrated that the presence of sinus rhythm was associated with a lower risk of death (HR 0.53; 95% CI [0.42–0.70]; p<0.001). However, this benefit was offset by the harm associated with the use of pharmacologic rhythm control (HR 1.50; 95% CI [1.18–1.89]; p<0.001).9
Catheter ablation has been shown in multiple randomised controlled trials to be superior to antiarrhythmic drug therapy in maintaining sinus rhythm when antiarrhythmic drugs have been ineffective, are contraindicated, or cause intolerable adverse effects.10 These percutaneous procedures are based on the electrical isolation of pulmonary veins, targeting the regions of the left atrium responsible for AF initiation and perpetuation. However, these second-line trials focused on patients who had already failed antiarrhythmic drug therapy. By design, these trials selected a population in whom antiarrhythmic drugs had already proven to be ineffectual, biasing the results towards a benefit for those patients randomised to catheter ablation. Until recently, it was not known whether early intervention (i.e. ablation performed prior to antiarrhythmic drug failure) would offer similar benefits in preventing arrhythmia, improving quality of life, and reducing healthcare utilisation.
Catheter Ablation as a First-line Therapy
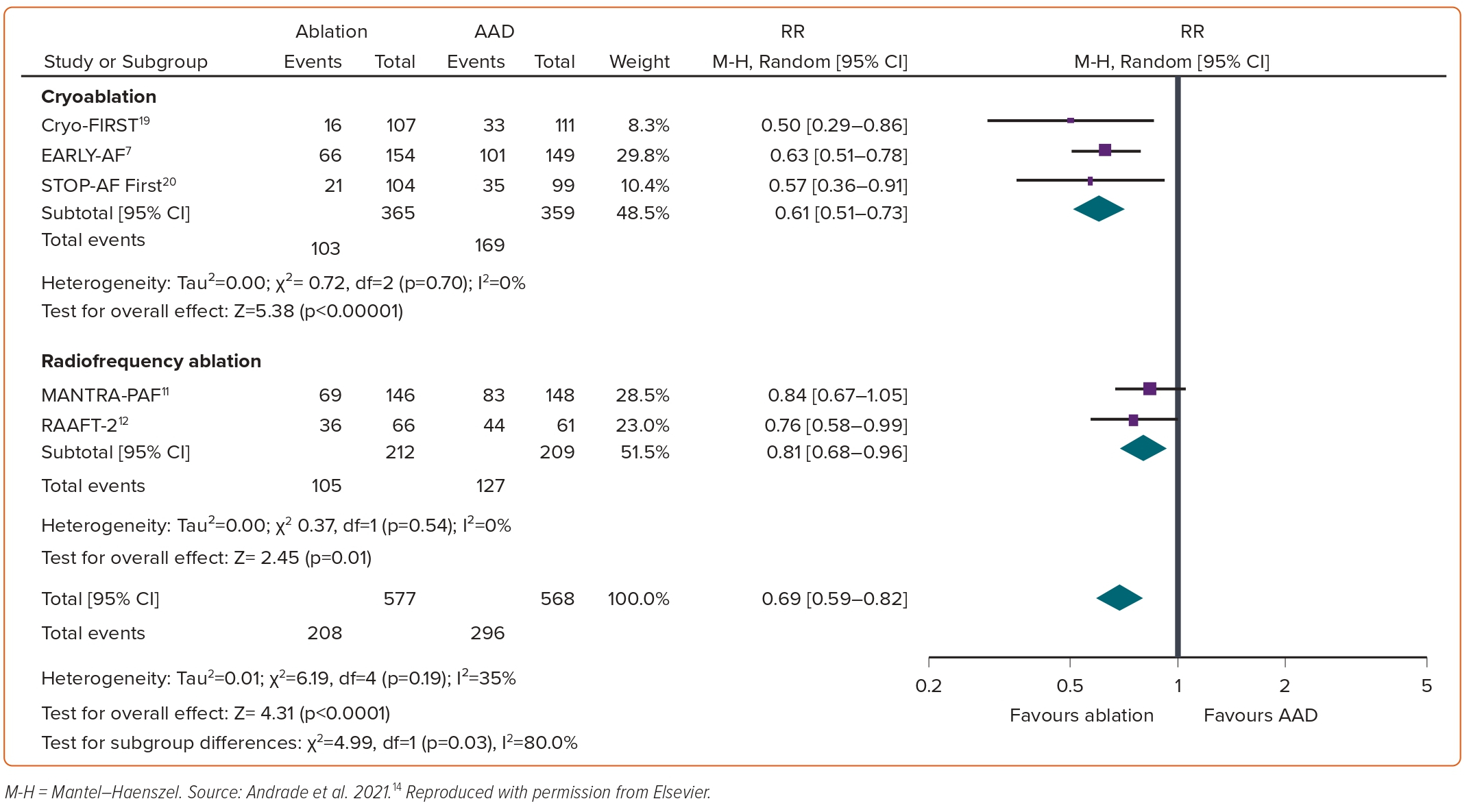
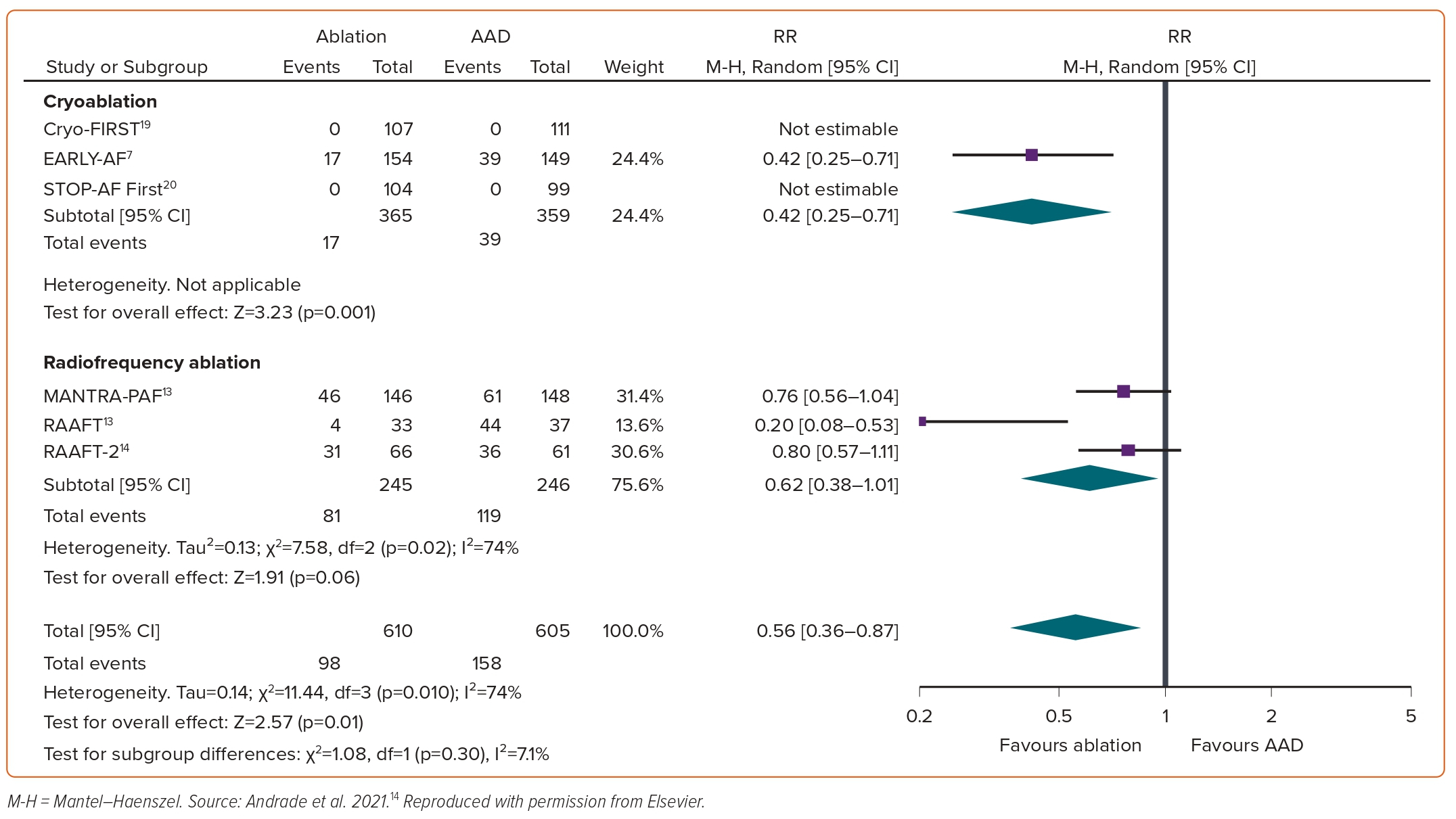
Between 2001 and 2010, there were three randomised trials of first-line focal point-by-point radiofrequency ablation: RAAFT-1, MANTRA-PAF and RAAFT-2.11–13 In aggregate, these three studies demonstrated that first-line ablation was more effective than antiarrhythmic drugs at preventing arrhythmia recurrence. However, the benefit was relatively limited (RR 0.81 for any arrhythmia; 95% CI [0.68–0.96]; p=0.01, Figure 1; and RR 0.62 for symptomatic arrhythmia; 95% CI [0.38–1.01]; p=0.06, Figure 2).14 These results did not translate into clinically meaningful improvements in quality of life or healthcare usage. Moreover, these studies were individually limited by relatively small sample size, high rates of crossover from antiarrhythmic drugs to ablation, lack of procedural standardisation, and differing procedural and study endpoints. As such, these studies had only a modest impact on clinical practice and did not result in a significant change to the guidelines.
The emergence of dedicated ablation technologies meant there was renewed interest in the role of catheter ablation as the initial management of treatment-naïve AF. These novel ablation technologies are less reliant on operator dexterity, enabling procedural standardisation and ensuring consistent outcomes with relatively low rates of complications.15–18
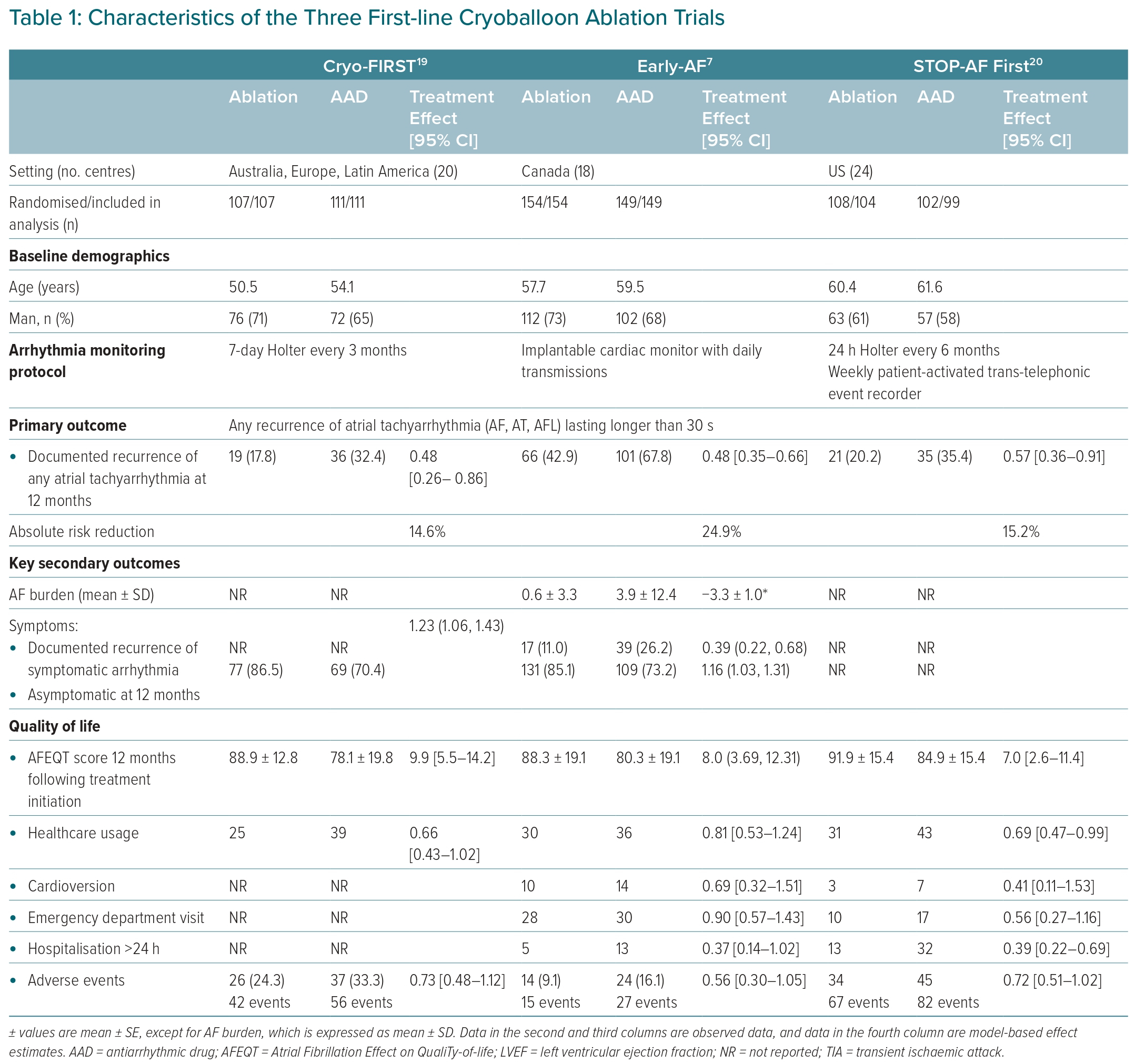
Initiated between 2014 and 2017, three multicentre parallel-group, single-blinded randomised clinical trials examined the role of first-line cryoballoon ablation: EARLY-AF, STOP-AF First, and Cryo-FIRST.7,19,20 The three trials included a total of 724 relatively young and relatively healthy patients with treatment-naïve paroxysmal AF in their intention-to-treat or modified intention-to-treat populations (Table 1).7,19,20
Cryoballoon Ablation as a First-line Therapy: 1-year Outcomes
Atrial Tachyarrhythmia Recurrence and Burden
The primary outcome for each of these studies was the first recurrence of any atrial tachyarrhythmia (defined as AF, atrial flutter, or atrial tachycardia) lasting 30 seconds or longer between 91 and 365 days after treatment initiation.
Documented recurrence of any atrial tachyarrhythmia occurred in 17.2–42.9% of patients randomised to cryoballoon ablation and 32.4–67.8% of patients randomised to antiarrhythmic drugs (AADs), with the reduction in the absolute rates of atrial tachyarrhythmia recurrence ranging from 15% (Cryo-FIRST and STOP-AF First) to 25% (EARLY-AF) at 1-year after treatment initiation. Despite differences in the intensity of arrhythmia monitoring, the relative benefit of first-line cryoablation was consistent between studies (HR 0.50 [0.29–0.86] in Cryo-FIRST, 0.57 [0.36–0.91] in STOP-AF First, and 0.63 [0.51–0.78] in EARLY-AF; pooled RR 0.61, 95% CI [0.51–0.73]).14,21
In addition, the EARLY-AF study demonstrated that the recurrence of symptomatic atrial tachyarrhythmia and AF burden (percentage time in AF) were significantly reduced with first-line ablation (absolute difference in symptomatic AF of 15.2%; RR 0.42, 95% CI [0.25–0.71]; number needed to treat [NNT] 7, and mean difference in AF burden of 3.3 ± 1.0% between the ablation and antiarrhythmic groups, respectively).7 In aggregate, 86% of patients randomised to ablation and 72% of patients randomised to antiarrhythmic drugs were free of AF-related symptoms at 1 year after treatment initiation (RR of being asymptomatic with ablation of 1.19; 95% CI [1.08–1.30]).7,19
Quality of Life
Patients enrolled in these studies had a significantly impaired quality of life at baseline (mean baseline Atrial Fibrillation Effect on QualiTy-of-Life [AFEQT] score of 60.1). At 1 year after treatment initiation, there was a significant improvement in health-related quality of life in both the ablation and the antiarrhythmic drug groups. However, patients randomised to catheter ablation achieved a significantly greater improvement in disease-specific and generic quality of life.7,14,19
Healthcare Usage
At 1 year after treatment initiation, significantly fewer patients randomised to first-line cryoballoon ablation experienced the composite healthcare usage outcome (RR 0.71; 95% CI [0.56–0.90]), with an absolute reduction of 9% (NNT 11).14 Significant reductions in all-cause hospitalisation (RR 0.38; 95% CI [0.23–0.63]), and numerical reductions in emergency department visits (RR 0.78, 95% CI [0.50–1.20]) and cardioversions (RR 0.60; 95% CI [0.31–1.18]) were also observed.14
Safety and Adverse Events
At 1 year of follow-up, clinically significant serious adverse events were comparable between patients randomised to first-line cryoballoon catheter ablation and antiarrhythmic drug therapy (RR 0.74; 95% CI [0.35–1.56]).14 However, with first-line cryoballoon ablation was associated with a slightly lower incidence of any adverse event at 1 year of follow-up (RR 0.70; 95% CI [0.54–0.89]).14
Cryoballoon Ablation as a First-line Therapy: Summary of 1-year Outcomes
Taken together, these three randomised studies demonstrated that an initial treatment strategy of cryoballoon catheter ablation for patients with treatment-naïve paroxysmal AF resulted in significant improvements in arrhythmia outcomes, produced clinically meaningful improvements in patient-reported outcomes, and significantly reduced healthcare resource usage, yet did not increase the risk of adverse events compared with initial antiarrhythmic drug therapy. This result was in contrast with contemporary guideline recommendations and strongly argued in favour of performing catheter ablation earlier.
The main limitation of these results was their relatively limited observation time. While the standard definition of ablation success involves an assessment of arrhythmia-free survival at 12 months post-ablation (a duration chosen based on the understanding that most recurrences transpire during this interval), this length of follow-up is inadequate when considering the impact of intervention on a relatively young population.22 Specifically, longer-term follow-up is required to provide information regarding the natural history of AF (e.g. disease progression), the longer-term durability of intervention on arrhythmic and patient-reported outcomes, as well as the downstream healthcare usage. In other words, a comprehensive assessment of longer-term clinical effectiveness is important to inform decision-making and enable patient empowerment regarding the choice of initial treatment, as well as for the evaluation of the cost-effectiveness of invasive AF ablation procedures.
Cryoballoon Ablation as a First-line Therapy: Longer-term Outcomes
The EARLY-AF study program was designed as a pragmatic multiphase platform to evaluate the effect of initial rhythm control treatment in patients with symptomatic treatment-naïve paroxysmal AF.23 The EARLY-AF trial intended to evaluate the effect of initial rhythm control treatment on atrial tachyarrhythmia recurrence and healthcare usage at 1 year of follow-up (the former being the guideline-recommended endpoint for AF ablation trials).7,22 The second phase, designated PROGRESSIVE-AF, was designed to evaluate the effect of initial rhythm control treatment on disease progression at 36 months of follow-up as assessed by an implantable continuous rhythm monitor.24
Consistent with 1-year results, at 36 months of follow-up there was a significantly lower rate of atrial tachyarrhythmia recurrence (56.5 versus 77.2%; HR 0.51; 95% CI [0.38–0.67]), and a significantly lower AF burden (mean difference in absolute AF burden −1.9 ± 0.7) observed in patients randomised to first-line cryoballoon catheter ablation.24
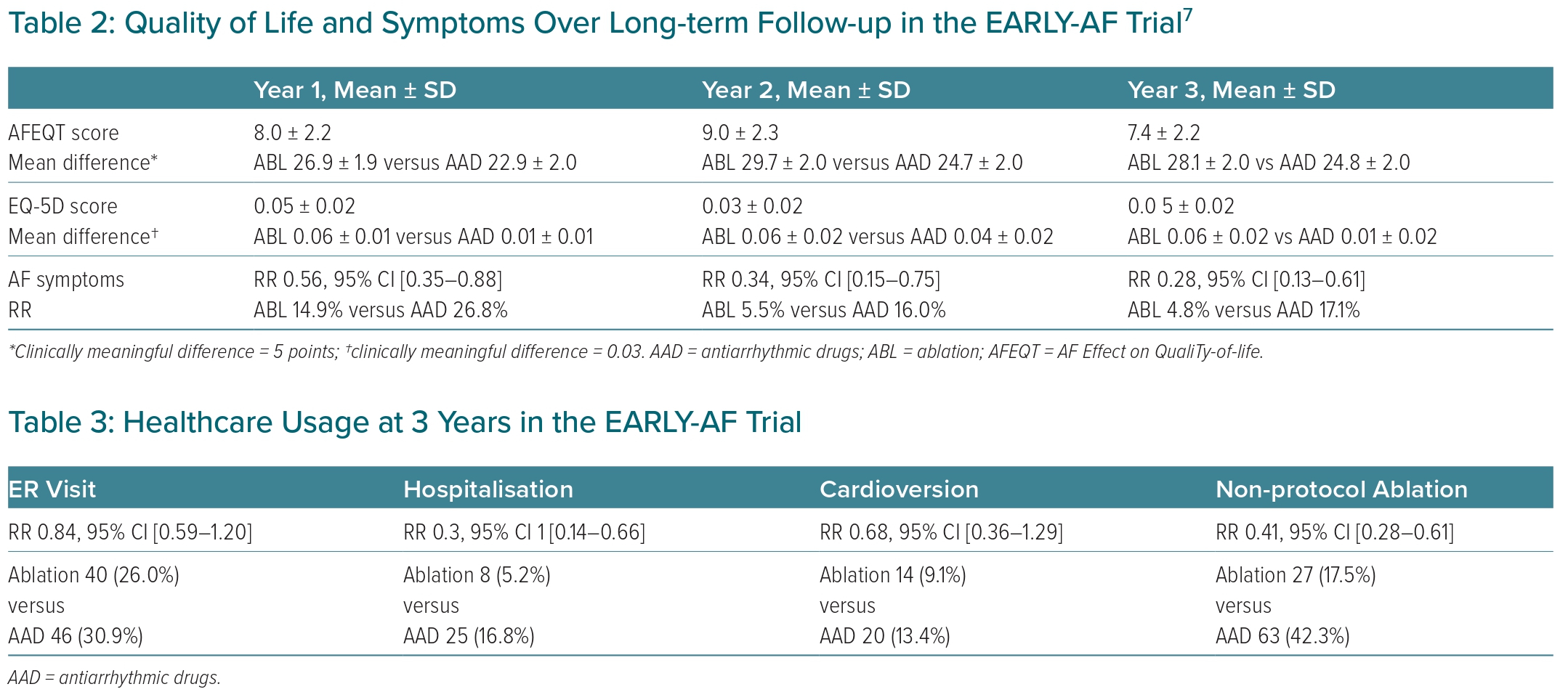
In addition, patients randomised to initial cryoballoon catheter ablation achieved significantly greater improvement in disease-specific quality of life (mean between-group difference in AFEQT score 7.4 ± 2.2 at 36 months), generic quality of life (mean between-group difference in EQ-5D score 0.05 ± 0.02 at 36 months), and were significantly less likely to report symptoms of AF at 36 months of follow-up (RR 0.28; 95% CI [0.13–0.61], Table 2).
Likewise, healthcare usage (Table 3) was significantly lower in patients randomised to initial cryoballoon catheter ablation. At 3 years, 5.2% of patients in the ablation group and 16.8% in the antiarrhythmic drug group had been hospitalised (RR 0.31; 95% CI [0.14–0.66]). Consistent with the 1-year outcomes, numerical reductions in emergency department visits and cardioversion were again observed in patients randomised to initial cryoballoon catheter ablation (RR 0.84; 95% CI [0.59–1.20] and RR 0.68; 95% CI [0.36–1.29], respectively).
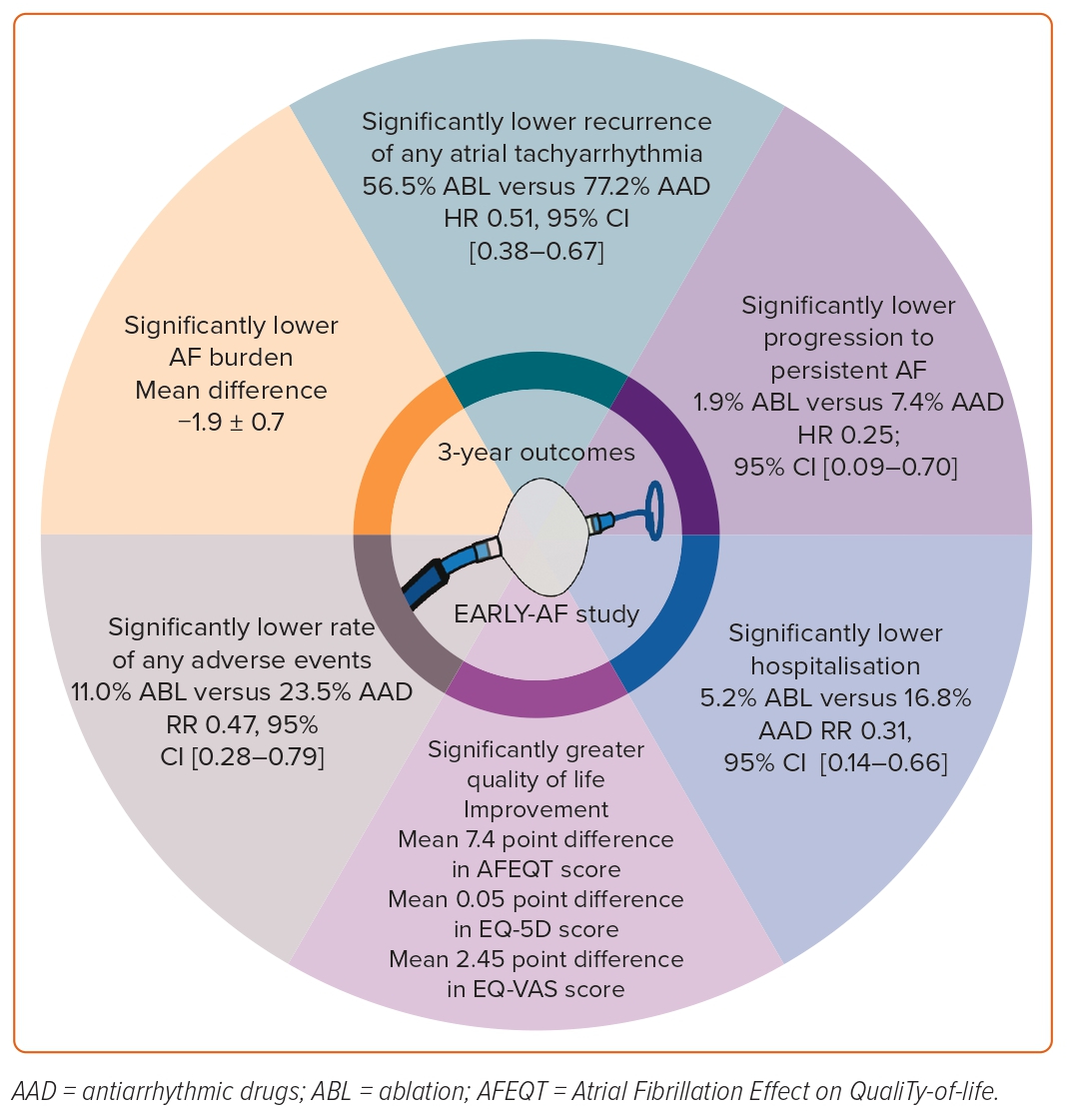
In contrast to the 1-year data, at 3 years adverse events were less likely to have occurred in patients randomised to initial cryoballoon catheter ablation (Figure 3). Over 3 years of follow-up, serious adverse events occurred in seven patients (4.5%) in the ablation group and 15 (10.1%) in the antiarrhythmic drug group, and any safety endpoint occurred in 17 patients (11.0%) in the ablation group and 35 (23.5%) in the antiarrhythmic drug group.
Cryoballoon Ablation as a First-Line Therapy: Disease Progression
While often categorised as paroxysmal and persistent forms, it is important to recognise that AF is a dynamic and chronic progressive disease. Initially, AF manifests as an isolated electrical disorder; however, electrical and structural atrial remodelling result in progression to a more sustained disorder. In contrast to antiarrhythmic drug therapy, catheter ablation is a personalised procedure designed to modify the mechanism of AF initiation and perpetuation through pulmonary venous isolation (e.g. trigger eradication), autonomic nervous system modulation (e.g. vagal denervation) and left atrial substrate modification (predominantly at the pulmonary venous–left atrial junction). As such, it was hypothesised that early invasive intervention could alter the progressive pathoanatomical changes associated with AF and, therefore, positively alter the disease trajectory.
In effect, this was what was observed in the long-term follow-up from EARLY-AF, whereby patients randomised to an initial strategy of catheter cryoballoon ablation experienced a lower incidence of persistent AF compared to those randomised to antiarrhythmic drugs (HR 0.25; 95% CI [0.09–0.70]), as determined by implantable continuous cardiac rhythm monitoring.24 Moreover, those who experienced an episode of persistent AF continued to experience progression in their disease, with the median duration of continuous AF episodes increasing from 15.8 days (8.0–88.2) to 54.4 days (11.4–163.8) by the end of study follow-up. Importantly, these findings were observed despite enrolling a relatively young population at objectively low risk of progression.
Unanswered Questions Regarding Ablation as First-line Therapy for AF
Can These Results be Extrapolated to Other Ablation Technologies?
As outlined above, previous studies evaluating the role of first-line focal point-by-point radiofrequency catheter ablation failed to demonstrate clinically meaningful improvements in arrhythmia outcomes, quality of life improvement, and healthcare usage.11–13 However, these studies were performed using previous-generation ablation technology. Since the publication of these trials, there has been an evolution in radiofrequency catheter technology, integrating a real-time quantitative estimation of contact between the tip of the catheter and the target myocardium. This ensures that the operator can assess the adequacy of catheter ablation electrode–tissue contact, which is a critical determinant of lesion quality. A recent multicentre randomised clinical trial has demonstrated comparative effectiveness for patients with AAD-refractory AF treated with cryoballoon ablation and contact-force guided radiofrequency ablation.25 However, an important observation is that the results observed with radiofrequency ablation are significantly associated with operator and centre experience, while cryoballoon ablation is associated with more consistent and reproducible procedural outcomes across a wider spectrum of operator and centre volumes.16 As such, in the absence of comparative trials, it may be reasonable to extrapolate the results of these first-line cryoballoon studies to contact-force guided radiofrequency ablation performed by experienced operators in high-volume centres. However, further study is required to determine if the results are applicable in lower volume centres.
A novel addition to these modern-generation catheter ablation technologies is pulsed-field ablation. This non-thermal ablation energy creates tissue injury through the delivery of a sequence of short-duration high-intensity electrical pulses. This high-intensity electric field induces a charge across the lipid bilayer, resulting in the formation of cell membrane pores (electroporation) that induce apoptotic cellular death. In contrast to radiofrequency and cryoablation, lesion formation with pulsed-field ablation is non-thermal and tissue-selective (e.g. cells exposed to an electric field strength above the critical tissue-dependent electric field threshold undergo irreversible electroporation, as above, and those exposed to a field below the critical threshold undergo reversible electroporation, whereby the pores close in time to re-establish homeostasis and regain viability). As such, it has been postulated that pulsed-field ablation may offer an improved safety and efficacy profile relative to thermal ablation energies, potentially positioning it as the preferred ablation energy for first-line AF ablation procedures.
How Early is Early Ablation?
The exact characterisation of early AF remains undefined, likely owing to the heterogeneity of presentation and subsequent prognosis. First-detected AF has been associated with increased short-term rates of death, stroke/systemic thromboembolism and major bleeding, suggesting that the occurrence of AF may be a marker of severe underlying comorbidity, or a consequence of worsening baseline disease.26
Conversely, in patients without significant comorbidity a significant proportion with first-detected AF will remain free of arrhythmia over prolonged follow-up (e.g. up to 50% of patients being free of arrhythmia recurrence in the absence of preventative treatment up to 5 years of follow-up).27–29 As such, a pragmatic definition may be to consider the definition employed in the aforementioned randomised clinical trials. EAST considered ‘early’ to be within the first year following diagnosis, which was similar to the median time from diagnosis to enrolment in the first-line cryoballoon catheter ablation trials.4,14 However, an important distinction is that the ablation trials (e.g. EARLY-AF) focused on a population of symptomatic patients necessitating intervention for the management of their AF (either pharmacological or catheter-based), whereas EAST included asymptomatic patients.
Ensuring Timely Access to Ablation
Once the decision to proceed to ablation has been made, it makes sense to ensure that it is performed in a timely manner. Observational data suggests that increasing time from diagnosis to ablation is associated with worsening clinical outcomes following the ablation procedure, including higher rates of arrhythmia recurrence, hospitalisation, heart failure decompensation, stroke and mortality.30–32 Unfortunately, many healthcare environments are constrained by lack of access to catheter ablation procedures, with procedural waitlists more than twice exceeding the recommended wait times.33,34 These delays have been associated with a significant number of patients progressing to more advanced forms of AF, leading to longer and more complex ablation procedures, as well as higher rates of peri-procedural complication and subsequent hospitalisation.35,36 As such, strategies to ensure timely access to intervention are essential when considering early intervention.
Conclusion
Ablation as a first-line therapy for AF is associated with significant reductions in arrhythmia recurrence, substantial improvements in arrhythmia-related symptoms and quality of life, and lower rates of adverse events. In addition, catheter ablation is associated with significantly lower rates of disease progression, suggesting that it is a disease-modifying intervention.