The concept of resistant hypertension (RH) has received great attention in the past years, mainly as a possible target population for innovative therapeutic strategies. An increasing number of original articles and commentaries have focused on the clinical significance, prognosis and treatment of this condition. Thus it is not surprising than even its definition is still a matter of debate, though the diagnosis of RH has important clinical implications, as patients with RH have an increased prevalence of secondary causes of hypertension,1 more severe target organ damage2 and increased cardiovascular morbidity and mortality risk of cardiovascular complications and death.3,4
According to the 2013 European Society of Hypertension/European Society of Cardiology (ESH/ESC) guidelines for the management of arterial hypertension, RH is a clinical situation in which blood pressure (BP) remains uncontrolled despite concomitant intake of at least three antihypertensive drugs (one of them preferably being a diuretic) at full doses.5 According to the 2008 American Heart Association (AHA) scientific statement, patients who require four drugs or more to have their BP controlled are also considered as resistant:6 this definition is probably the most used nowadays, but has several shortcomings, which will be discussed below.
First, it should be noted that definition of RH is substantially different from the one of uncontrolled BP. A recent cross-sectional cohort study compared the clinical characteristics of RH patients with uncontrolled BP and with controlled BP (those that are considered RH by AHA but not by ESC/ESH guidelines) and found many similarities.7 However, RH patients with uncontrolled BP showed higher prevalence of diabetes and increased low-density lipoprotein (LDL) cholesterol levels.7 An increased prevalence of associated risk factors might thus be associated with a worse prognosis.
However, the main caveat in current definitions of RH is probably the fact that is still based on office rather than out-of-office BP pressure measurement. For that reason, it has been recently proposed that a new, stricter definition of RH based on ambulatory BP measurement might be more useful in risk stratification and in patients selection for device-based therapies.8
Indeed, white-coat resistant hypertension might be responsible for about one-third of the classically defined RH patients, as highlighted by de la Sierra and co-authors in a cohort consisting of 8,295 RH patients from the Spanish Ambulatory Blood Pressure Monitoring Registry. True RH patients were younger, more frequently men, with a longer duration of hypertension and a worse cardiovascular risk profile, including a greater prevalence of associated risk factors, of cardiac and renal target organ damage and of established cardiovascular disease, compared with white-coat RH patients.2 Interestingly, in the same study the prevalence of masked RH (uncontrolled ambulatory BP but controlled office BP) was 31.0 % among hypertensive patients taking three antihypertensive drugs, confirming that the use of office or 24-h BP for the definition of RH identifies two populations that only partially overlap. A higher cardiovascular risk in true RH patients was confirmed by a prospective study recruiting 742 treated hypertensive patients followed up for about 5 years.9 In this study, the rate of cardiovascular fatal and nonfatal events was twofold in masked RH patients and almost threefold in true RH patients compared with responders to treatment, while it was not increased in white-coat RH patients. Furthermore, Salles et al. demonstrated that ambulatory BP, especially nighttime BP, but not office BP, predicts cardiovascular events and overall death in a cohort of 556 apparently RH patients.10
Resistant Hypertension: Which Prevalence?
Given the discrepancies among definitions of RH and the ongoing debate within the scientific community, it is not surprising that several uncertainties about the prevalence of RH exist. In the general outpatient population with newly diagnosed hypertension, the incidence of RH is very low, ranging from 1 % to 2 % over a median 1.5 years.4 The prevalence of RH is expected to increase in the next years, due to increased life expectancy and increased prevalence of obesity, diabetes and chronic kidney disease, all factors associated with difficult-to-control hypertension. In the National Health and Nutrition Examination Survey (NHANES) study, the prevalence of apparent RH increased from 15.9 % (1998–2004) to 28.0 % (2005–2008) of treated hypertensive patients, confirming this trend.11 RH can be even more common in selected populations at high cardiovascular risk or in clinical trials where forced up-titration of antihypertensive drugs occurs.
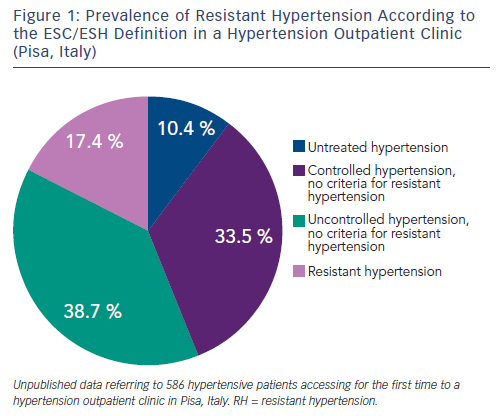
For example, in the Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT), which enrolled about 33,000 hypertensive participants with associated cardiovascular risk factors, 27 % required at least three BP medications.12 Similarly, in a subanalysis of the International Verapamil-Trandolapril Study (INVEST), which included 17,190 patients with established coronary artery disease, the prevalence of RH was 38 %.13 In hypertension specialty clinics, where up-titration of antihypertensive medications occurs regularly and more severe cases are referred to, the prevalence of RH may range between 12 and 40 % (see Figure 1).14,15 Among the 10,700 participants of the Reasons for Geographic and Racial Differences in Stroke study on treatment for hypertension, the prevalence of RH was double in the presence of an estimated glomerular filtration rate (GFR) lower than 45 ml/min per 1.73 mq, in comparison to individuals with GFR greater than 60 ml/min per 1.73 mq.16 A recent systematic review and meta-analysis exploring the prevalence of RH in treated hypertensive populations included 20 observational studies and four randomised control trials (RCTs) with a total population of 961,035 individuals. The random-effect method for observational studies and RCTs yielded RH prevalence ratios of 13.72 % and 16.32 %, respectively. However the authors noted that most studies were incapable of ruling out pseudoresistance caused by white-coat effect, poor medication adherence and suboptimal dosing.17
Hypertension: Which Treatment?
One of the main reasons for the lack of efficacy of antihypertensive pharmacological treatment, and for pseudoresistant hypertension, is that very often drugs are not administered at the correct dosage and in rational combinations.18 This is especially the case for angiotensinconverting enzyme (ACE) inhibitors, compounds characterised by a flat dose-response curve. The significance of this flat doseresponse curve is that a low dose of an ACE inhibitor has the same antihypertensive potency as a high dose but a shorter duration of action.19 Since the prescription of inadequate drug dosages and/ or inadequate drug combinations are probably among the main and overlooked causes of pseudoresistance to antihypertensive treatment, it is therefore important to be aware of the clinical pharmacology of antihypertensive drugs in order to choose not only the class or the molecule best suited to the clinical characteristics of the patient, but also the correct dosages and combinations, in order to ensure effective and homogeneous 24-h BP reduction.19 Garg and coauthors demonstrated that 58 % of the patients referred to a tertiary care hypertension clinic for uncontrolled BP by three or more BP-lowering drugs had incorrect drug dosage and/or combination.18 More recently, this aspect has been elegantly taken into account in the design of the Renal Denervation for Hypertension (DENERHTN) study, a nationwide, multicentre, open-label, Q3 RCT testing the effect of a standardised stepped-care antihypertensive treatment alone or plus renal denervation in RH patients.20 Of 121 eligible patients (in which secondary hypertension had been excluded), 12 had their ambulatory BP controlled after switching to a standardised triple therapy (indapamide 1.5 mg, ramipril 10 mg or irbesartan 300 mg and amlodipine 10 mg daily), and thus were not randomised to treatment.20 Furthermore, there is evidence to suggest that monotherapy with renin-angiotensin system (RAS) blockers in low-renin individuals might induce a pressor response in a not negligible proportion of individuals.21 The impact of this poorly studied phenomenon on individuals in combination therapy such as RH is still unknown.
Improving treatment compliance is another crucial therapeutic target in RH patients. Non-adherence to BP-lowering therapy, detected by high performance liquid chromatography-tandem mass spectrometry urine analysis, is common, particularly in patients with suboptimal BP control and those referred for renal denervation to a tertiary hypertension clinic.22 Ambulatory BP monitoring after witnessed drug intake might also be a more-feasible and less-expensive option.23 An increasing body of evidence suggests that the use of drug monitoring in RH might be useful not only for diagnostic purposes, in order to assess actual compliance to antihypertensive treatment, but also for therapeutic purposes, as recently supported by preliminary observations.24 There is still a paucity of data about how to improve compliance to non-pharmacological and pharmacological treatment in resistant hypertensive patients. In this view, hypertension specialists should be careful not to negatively label the non-compliant patient, whereas the use of therapeutic drug monitoring results should always be accompanied by counselling of methods to overcome barriers to adherence.
An adequate clinical workup in RH patients must include a thorough screening for secondary causes of hypertension, which are conceivably more frequent in this subgroup of individuals in comparison to the general hypertensive population.25 Obstructive sleep apnoea syndrome (OSAS) and primary aldosteronism are particularly common in RH patients.1,26 A large retrospective study analysed a group of 1,616 patients with RH over 20 years and demonstrated that 20 % resulted to have a positive aldosterone–renin ratio, and after three confirmatory tests the diagnosis of primary aldosteronism was confirmed in 11.3 %.1,26 By contrast, the ‘spontaneous hypokalemia/no antihypertensive drug’ diagnostic approach resulted in predicted primary aldosteronism prevalence rates of less than 0.5 % of hypertensive patients,26 thus hyperaldosteronism should be actively searched for in RH patients. It should also be noted that aldosterone-antagonists, such as spironolactone, are the most effective BP-lowering drugs that can be used in RH patients on top of three drugs (usually a RAS-blocker, a simil-thiazide diuretic and a calcium-channel blocker).
This was suggested first from the non-randomised post hoc analysis of the Anglo-Scandinavian Cardiac Outcomes Trial-Blood Pressure Lowering Arm, in which the addition of spironolactone to a triple-drug treatment led to a significant decrease of systolic BP of 21.9 mmHg and diastolic BP of 9.5 mmHg.27 The beneficial effect of add-on therapy with spironolactone 25 mg against placebo in RH patients was then confirmed in the Addition of Spironolactone in Patients with Resistant Arterial Hypertension (ASPIRANT) study, an investigator-led, prospective, multicentre, randomised, double-blind, placebo-controlled, parallel-group trial,28 demonstrating a significant difference in mean fall of systolic BP on daytime ambulatory BP monitoring of 5.4 mmHg between the two arms. Finally, the Prevention And Treatment of Hypertension With Algorithm-based therapy number 2 (PATHWAY-2) study was a double-blind, placebo-controlled, crossover trial, enrolling 335 patients with office and home BP uncontrolled despite treatment for at least 3 months with maximally tolerated doses of three drugs. Patients rotated, in a pre-assigned, randomised order, through 12 weeks of once-daily treatment with each of spironolactone (25–50 mg), bisoprolol (5–10 mg), doxazosin-modified release (4–8 mg) and placebo, in addition to their baseline antihypertensive drugs. Spironolactone was the most effective drug in lowering home systolic BP compared with placebo (–8.70 mmHg), but also compared with the other two active treatments.29 Spironolactone was more effective in the presence of low plasma renin levels, suggesting that among underlying pathophysiological causes of RH, sodium retention and undetected aldosterone producing adenomas play a significant role. Based on the results of the PATHWAY-2 study, the authors claim that truly RH should now be considered rare and redefined as BP not controlled by three drugs at full doses (a RAS-blocker, a simil-thiazide diuretic and a calcium-channel blocker), plus spironolactone. The role of sodium retention in pathogenesis of RH is also reinforced by the striking effect of dietary sodium restriction in this population. In a small 4-week, randomised, crossover trial, 12 RH patients were randomised to the low- (50 mmol of sodium per day) or high-salt diet (250 mmol of sodium per day) for 1 week. The difference in mean BP between the two treatments was –20.1 mmHg for systolic BP and –9.8 mmHg for diastolic BP.30
OSAS is commonly associated with hypertension31–33 and with RH, with a prevalence reaching up to 60 % in the latter group.1 Normotensive individuals with OSAS and increased daily sleepiness have an increased risk of developing hypertension, while continuous positive airway pressure (CPAP) is able to reduce its incidence.33 Based on this evidence, some authors claim that OSAS should be considered among secondary causes of hypertension.1,25 This point of view is reinforced by the fact that the treatment of OSAS by CPAP is able to effectively reduce ambulatory BP valueseven in RH patients.34 Furthermore, it is likely that any cause of sleep loss and fragmentation may be associated with increased BP values and RH,35,36 though the actual prevalence of other sleep disorders in RH and the impact of their presence and treatment on BP control have not been investigated yet.
In the past years, device-based therapies targeting sympathetic activation, namely renal denervation and baroreceptor activating therapy, have been tested in RH patients.37 After brilliant results in proof-of-concept, small studies, both the techniques failed to demonstrate sustained efficacy in large randomised trials.34,35,38,39 As demonstrated by the results of the DENERHTN trial,20 which showed a significant (and realistic) drop in ambulatory BP (mean adjusted difference in daytime systolic BP −5.9 mm Hg) in resistant hypertensive patients after renal denervation on top of a rational, standardised titration and add-on therapeutic scheme, an accurate definition of RH is crucial in order to select patients for invasive and expensive therapies.
In conclusion, the debate on definition and treatment of RH is still open. The first, broader definitions of RH still retain an utility in identifying the subset of individuals requiring special attention by the clinician: in this subset of individuals, the hypertension specialist should be involved in order to exclude spurious forms of RH, including whitecoat RH, inappropriate therapeutic schemes and lack of adherence to treatment. Only the remaining individuals, those with true RH, should then undergo an extensive diagnostic workup for secondary causes of hypertension and other conditions whose removal might represent a benefit for BP control and then offered with innovative therapies if appropriate. In this view, RH can be still considered a special entity requiring special treatment.
Conclusions
RH was defined many years ago as a clinical situation in which BP remains uncontrolled despite concomitant intake of at least three antihypertensive drugs (one of them preferably being a diuretic) at full doses. The broader, original definition should be used as a temporary label, useful for the identification of a subset of hypertensive patients requiring a more extensive clinical workup in order to achieve an adequate BP control, but does not represent per se a separate clinical entity requiring special treatment. This definition allowed a substantial proportion of patients with white-coat RH or suboptimal medical treatment to undergo, for example, renal denervation, while pseudoresistance to treatment might be responsible to about 95 % of cases of apparently RH.18
Conversely, a more stringent definition would require, as a minimum set of additive criteria the confirmation of uncontrolled BP by means of ambulatory BP monitoring, the verification of a rational combination therapy and the failure of add-on therapy with spironolactone; objective assessment of treatment compliance and a thorough screening for secondary causes of hypertension, followed by tailored treatment, should be implemented too. This approach will reduce the number of residual uncontrolled patients and will help improve BP control in the hypertensive population, possibly reducing also healthcare costs.







