Atrial fibrillation (AF) is the most commonly encountered arrhythmia in clinical practice in Western countries. The prevalence of AF depends on the population studied1 and especially on age.2–9 It is affected by increasing longevity and is modulated by the prevalence of cardiovascular risk factors, especially arterial hypertension and related habits. In Spain, for example, the prevalence of AF among people >40 years of age is about 4.4 %,9 rising to 8.5 % among those >60 years and reaching 16.5 % among those >85 years.4 The prevalence of AF is expected to double in the next 50 years.10,11
AF is characterised by the anarchic (fast and disorganised) and unpredictable contraction of atrial muscle fibres. This arrhythmia is appears on an electrocardiogram as an absence of P-waves and irregular R-R intervals. It is usually associated with tachycardia. The resulting asynchrony leads to ineffective contraction, decreased ventricular ejection fraction and blood pooling, predisposing to coagulation inside the atrium and increasing the risk of thromboembolic events.
AF increases the risk of mortality and morbidity, resulting in high healthcare costs. It increases the probability of stroke by two- to sixfold and the probability of death by 1.5-fold to 2.2-fold.12–18 Moreover, the risk of stroke recurrence is higher in patients with AF than in those without. AF has been also associated with cognitive dysfunction, diminished quality of life and diminished functional capacity.19–22
The prevention and treatment of AF is important for both patients and healthcare systems. The complexity of the mechanisms involved calls for a multidimensional approach. Since AF is potentially dangerous,efforts to correct and/or control it are required; however, for various reasons these efforts often fall short. The efficacy of antiarrhythmic drugs is unpredictable, depending mainly on the duration of AF and the patient’s underlying heart disease. Moreover, antiarrhythmic drug treatment can cause proarrhythmia. AF can also recur after catheter ablation. Given the uncertain success of attempts to directly treat AF, it is therefore important to manage the attendant increased risk of thromboembolic events; most patients with AF will eventually need anticoagulant therapy to prevent thromboembolism.21,23,24
During the past 50 years, vitamin K antagonists (VKAs) have become the first-line oral anticoagulant treatment of choice for preventing thromboembolic events.25 Although VKAs improve prognosis by reducing thromboembolic events, they have diverse clinical limitations (see Figure 1).1 VKAs significantly increase the risk of minor and major bleeding complications, of which intracranial haemorrhage is particularly harmful. They can interact with many drugs and foods, and their effects are also influenced by hepatic metabolism. Regular monitoring and dose adjustments are thus essential to keep patients within the narrow therapeutic range throughout VKA treatment.
The novel direct-acting oral anticoagulants (DOACs) such as dabigatran, rivaroxaban, apixaban and edoxaban have been developed to overcome the limitations of VKAs and are now considered a valid alternative.21 Compared to VKAs, DOACs are at least as effective in reducing stroke and systemic embolism and are associated with a lower risk of haemorrhage.26–29 Another benefit is their predictable, dose-related effects that do not require close monitoring.26–30 The beneficial effects of DOACs over VKAs have been documented in several subsets of patients with AF including patients with diabetes mellitus, with heart failure and with previous stroke.31
Until recently, the main drawback of novel anticoagulants was the lack of an agent to reverse their effects. Now, however, various clinically-effective antidotes have been developed.32–35 Another factor against the use of DOACs is their cost. Although they are more expensive than VKAs, comparisons of the overall costs of the two treatment strategies in various contexts have demonstrated that DOACs can be a cost-effective alternative to dose-adjusted warfarin for stroke prevention in AF in most patients.36–41 For these reasons, the European Society of Cardiology and several other scientific societies now recommend using DOACs as first-line therapy.23,42,43
This review presents the current recommendations for the use of DOACs in patients with nonvalvular AF at high risk of bleeding.
Stratifying the Risk of Thromboembolism and Bleeding in AF
All anticoagulants increase the risk of bleeding. The benefits of decreasing the risk of thromboembolism must be weighed against the potential harm of increasing the risk of bleeding. Several scores have been proposed to assess these risks. Currently, the most widely recommended and used scores are the CHA2DS2-VASc (Congestive heart failure, Hypertension, Age >75 years, Diabetes, prior Stroke/transient ischemic attack, Vascular disease, Age 65–74 years, Sex category) for thromboembolism and the HAS-BLED (Hypertension, Abnormal renal/liver function, Stroke, Bleeding history of/predisposition, Labile international normalised ratio (INR), Elderly, Drug therapy/alcohol intake) for bleeding. These scores have been validated in very broad populations.44–48
The European Society of Cardiology has proposed an algorithm for managing thromboembolic and haemorrhagic risk in patients with AF.23 Other strategies for reducing thromboembolic risk include aspirin and antiplatelet drugs; however, compared to anticoagulation alone, aspirin alone provokes similar indexes of intracranial bleeding and higher rates of other major bleeding events.47 There is thus no argument for using aspirin instead of anticoagulation because of bleeding risk.49 Aspirin plus antiplatelet therapy or, less effectively, aspirin alone may, however, be considered in patients who refuse oral anticoagulant treatment. Importantly, the HAS-BLED score should not be used to exclude patients from treatment; rather it should be used to correct potentially reversible risk factors and to determine whether selected patients with the highest risk of bleeding could benefit from low doses of DOACs. Chao et al. recently analysed the risk of stroke in 186,570 patients with AF not using antiplatelet or anticoagulant agents to determine whether patients with a single risk factor (apart from sex) should receive oral anticoagulation.50 Analysing the impact of the components of the CHA2DS2-VASc score, they found that the weight of the components differed; the risk of stroke was highest for age, followed by the presence of diabetes mellitus. Thus, given the high risk of ischaemic stroke, oral anticoagulation is recommended in all patients with CHA2DS2-VASc scores greater than two and in most patients with CHA2DS2-VASc scores of one, unless the only risk factor is female sex.49,50
Results of Pivotal Clinical Trials Using DOACs
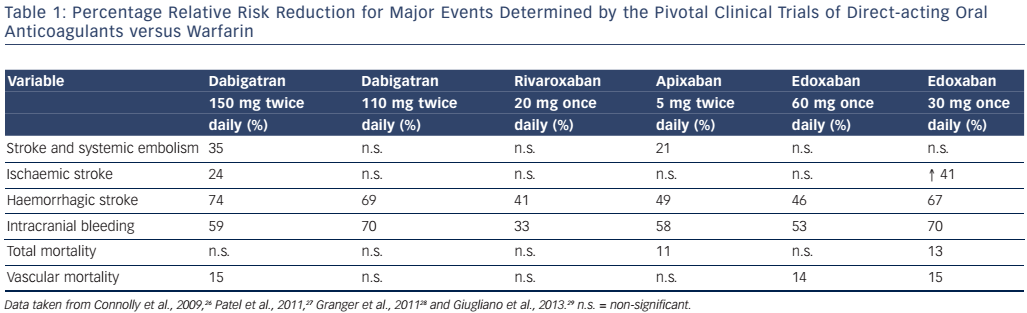
To date, four extensive randomised clinical trials comparing four DOACs (dabigatran, rivaroxaban, apixaban and edoxaban) with warfarin in different cohorts of patients with nonvalvular AF have been published.26–29Table 1 summarises the results of these trials, which have led to the authorisation of these DOACs for clinical use in AF in several countries. Since the publication of these trials, the results of two large observational studies that equalled or surpassed the clinical results obtained in the clinical trials have been published, further supporting the use of DOACs.51,52 The efficacy and safety of DOACs can thus be considered as good as or possibly better than those of VKAs,26–29,53 and DOACs have the additional advantage that their effects are dose-dependent and predictable. Furthermore, the advantages of DOACs over VKAs have been demonstrated in several specific groups of patients with AF (e.g. patients of both genders or with comorbidities such as heart failure, arterial hypertension, diabetes mellitus and previous stroke).31
Monitoring of DOACs
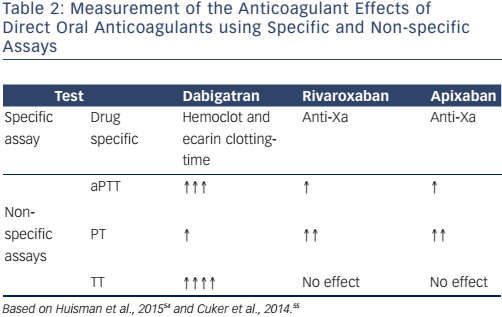
Although routine monitoring of coagulation levels is not necessary in patients on DOACs, simple and widely-available tests (see Table 2) help measure their anticoagulant effects if unexpected situations, such as urgent surgery, haemorrhagic events, overdose or acute renal failure, require it.
Dabigatran prolongs the activated partial thromboplastin time (aPTT), but this effect is not linear and the sensitivity of aPTT reagents varies greatly. A trough aPTT (>12 hours after the most recent dose) >80 seconds or two- to three-times higher than the baseline value is associated with a higher risk of bleeding, whereas a normal aPTT indicates that dabigatran has no clinically-significant anticoagulant effect.54 A normal thrombin time (TT) is an indicator of a drug concentration outside the clinically-relevant range.55 The ecarin clotting time (ECT) measures dabigatran activity and the diluted thrombin time with an appropriate dabigatran calibrator (Hemoclot® thrombin inhibitor assay) measures dabigatran concentration. Dabigatran plasma concentration >200 ng/ml or an ECT three to four times the baseline value or >65 seconds at trough is associated with increased bleeding risk.54 Prothrombin time (PT) and INR are not useful for measuring dabigatran’s effects.56
PT is of limited value for monitoring the anti-Xa anticoagulants rivaroxaban, apixaban and edoxaban. Rivaroxaban and apixaban may prolong PT, but PT is highly dependent on the reagent used in the assay.55–57 A normal PT, however, indicates that these drugs are not having a clinically-significant effect. PT, aPTT and INR should not be used to measure edoxaban’s effects due to this lack of evidence, presumed insensitivity, the significant variation between reagents and lack of standardisation, which also effect the measurements of other direct anti-Xa inhibitors.56 Anti-factor Xa assays using rivaroxaban, apixaban and edoxaban standards do, however, provide accurate information and seem the best approach to quantifying the anticoagulant effects of these drugs.55,57
Reversal of DOAC effects
To reverse the effects of a DOAC, it is essential to know the type of DOAC administered, the dosing regimen, the time since the last dose was administered and factors influencing plasma concentration, e.g.renal failure. Time reduces the effects of anticoagulants. Currentlyavailable DOACs have short half-lives (about 12–15 hours), and their effects would be expected to completely disappear by four drug half-lives (after about 48–60 hours).58 DOACs are absorbed and have an anticoagulant effect 1–4 hours after consumption, so early gastric lavage can be considered if little time has elapsed since the last dose. The administration of oral activated charcoal is useful within 2 hours of dabigatran intake and within 6 hours of apixaban intake.58 The clearance of all DOACs depends to varying extents on renal function, so adequate hydration and diuresis are essential. Haemodialysis can be used for the emergency elimination of dabigatran; however, the risk of bleeding at puncture sites for dialysis needs to be carefully balanced against the risk of waiting. Nonspecific procoagulant agents (prothrombin complex concentrates and activated factor VIIa) have been used to treat serious bleeding, but the results are controversial.58,59
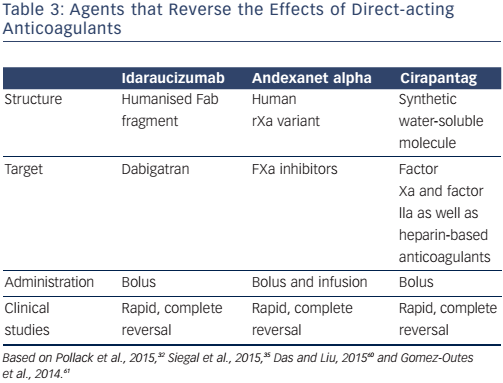
The recent advent of target-specific reversal agents that enable the effects of DOACs to be reversed within a few minutes (Table 3) represents a major safety advance in urgent situations.32–35,60 One such agent, idarucizumab, is a humanised monoclonal antibody fragment with 350 times higher affinity for dabigatran than thrombin but lacks thrombin-like enzymatic activity and does not bind thrombin substrates.60 It is easily administered intravenously (5 g as two 50-ml bolus infusions, no more than 15 minutes apart) and specifically and completely reverses the anticoagulant effects of dabigatran in few minutes.Ex vivo studies in rats have shown that steady-state dabigatran levels of 200 ng are completely reversed within 1 minute after the administration of an intravenous bolus of idarucizumab.60 The safety and efficacy of idarucizumab have also been demonstrated in patients requiring urgent procedures or presenting with severe bleeding.32 This drug is available for clinical use in some countries, obviating the need for dialysis in emergencies.32,33
Another target-specific reversal agent, andexanet alfa, has been designed specifically to reverse the anticoagulant effects of factor Xa inhibitors.34,35,60 Andexanet alfa is a recombinant modified decoy of factor Xa. Its efficacy has been demonstrated in healthy volunteers treated with apixaban or rivaroxaban; it reverts anticoagulant activity within minutes after administration and for the duration of infusion.35 In these healthy volunteers, transient increases in D-dimer and prothrombin fragments 1 and 2 without clinical thrombotic events have been observed. The dose of andexanet alfa depends on the DOAC. Whereas a 400-mg intravenous bolus followed by a continuous infusion of 4 mg/min for 120 minutes reverses the effects of 5 mg of apixaban twice daily, reversing the effects of 20 mg of rivaroxaban once daily requires 800 mg as an intravenous bolus (30 mg/min) followed by continuous infusion of 8 mg/min for 120 minutes.35
PER977, also called aripizine or ciraparantag, can also reverse the effects of factor Xa inhibitors. This small, synthetic, water-soluble molecule binds to direct inhibitors of factor Xa and factor IIa as well as to heparin-based anticoagulants. It antagonises the effects of all anticoagulants except VKAs and argatroban within 30 minutes after intravenous administration, and has a clearance half-life of about 1.5 hours.60,61 To date, however, very few clinical data have been published62 and the drug is not yet clinically and commercially available.
Periprocedural Management of Patients Treated with DOACs
One of the most important issues related to DOACs in daily clinical practice is appropriate periprocedural management to reduce the risk of bleeding events and the inherent risk of thromboembolic events. This challenge encompasses a wide range of clinical scenarios, including elective and urgent surgery as well as circumstances involving the risk of fatal haemorrhage, such as multiple traumas.
The first step in the periprocedural management of a patient on a DOAC is to determine the risks of thromboembolism with the CHADS-VASc score and bleeding with the HAS-BLED score.23,24 Next, the inherent risk of bleeding associated with the invasive procedure to be undertaken must be determined and weighed against the benefit of remaining on anticoagulants on a case-by-case basis. Clinical guidelines detailing the risks involved in different invasive procedures and recommendations to minimise them63,64 have proven very useful in clinical practice.65
The decision to continue or to pause anticoagulant treatment should be based on pharmacokinetic principles and the estimated thromboembolic and bleeding risks. Interestingly, accumulating evidence is leading to a consensus that bridging with heparin is unnecessary in patients treated with a DOAC64–66 and that the availability of fast-acting reversal agents minimises anticoagulant-related bleeding during urgent or emergent interventions.
Conclusion
AF is very common and is associated with increased morbidity, mortality and healthcare costs. Appropriate clinical management, including the prevention of thromboembolic events, is thus crucial. Preventing thromboembolic events with VKAs has various clinical limitations; DOACs overcome these limitations and have proven efficacious and safe. The recent developments of tests that allow the monitoring of anticoagulant levels and of target-specific reversal agents for DOACs have facilitated the use of these drugs in several situations, including emergencies.