Definition and Epidemiology
Congestive heart failure is a clinical syndrome that can result from any structural or functional cardiac disorder that leads to failure of the heart to deliver oxygen at a rate commensurate with the requirements of the metabolising tissues. The main symptoms are dyspnoea and fatigue, which limit exercise capacity, and there is fluid retention and elevated filling pressures, which can lead to pulmonary vascular congestion or peripheral oedema. In addition, there is typically cardiac dilatation and reduced left ventricular (LV) systolic function. Over the last two decades this concept has been challenged by studies which show that up to 50 % of patients with congestive heart failure have normal or preserved LV ejection fraction (LVEF).1–5 It appears that in most of these patients the heart failure is due to abnormal diastolic function.6 However, other cardiac disorders may also cause heart failure with normal LVEF, including right ventricular failure, valvular heart disease and pericardial disease. When abnormal diastolic function can be identified, the condition has been described as diastolic heart failure.7 There has been some controversy, however, regarding the use of the name diastolic heart failure. This controversy is in part due to problems with measuring diastolic function in clinical routine.8 In addition, there are studies which suggest that patients with heart failure with preserved left ventricular ejection fraction (HF-PEF) may actually have reduced systolic function.9 It can also be argued that the differentiation into systolic and diastolic heart failure is somewhat artificial since patients with systolic heart failure also have diastolic dysfunction.
As a consequence, a terminology based upon measurement of LVEF is frequently used, and patients are grouped into heart failure with reduced ejection fraction (HF-REF) when EF is <50 % or HF-PEF when EF is ≥50 %.1 In some clinical trials the HF-PEF group has been expanded to include patients with mild systolic heart failure and the limit is set to EF >40–45 %.10,11 Whereas this may be practical in a clinical trial, we do not recommend such a wide definition when discussing mechanisms of heart failure since the inclusion of patients with obvious reduction in systolic function may confuse the issue. A limitation of these definitions based on EF is that they may put too much emphasis on EF, which is a suboptimal measure of LV function. Thus none of the terminologies discussed above are perfect, and at the present time both HF-PEF and diastolic heart failure are in use. In this article we define HF-PEF as heart failure with EF ≥50 % and we use the term diastolic heart failure only when referring to patients with impaired diastolic function.
HF-PEF is relatively uncommon in younger patients, but increases in importance in the elderly and the frequency is higher in females than in males and is associated with a history of hypertension.12 The trend is that the proportion of heart failure patients who have preserved EF is increasing.5
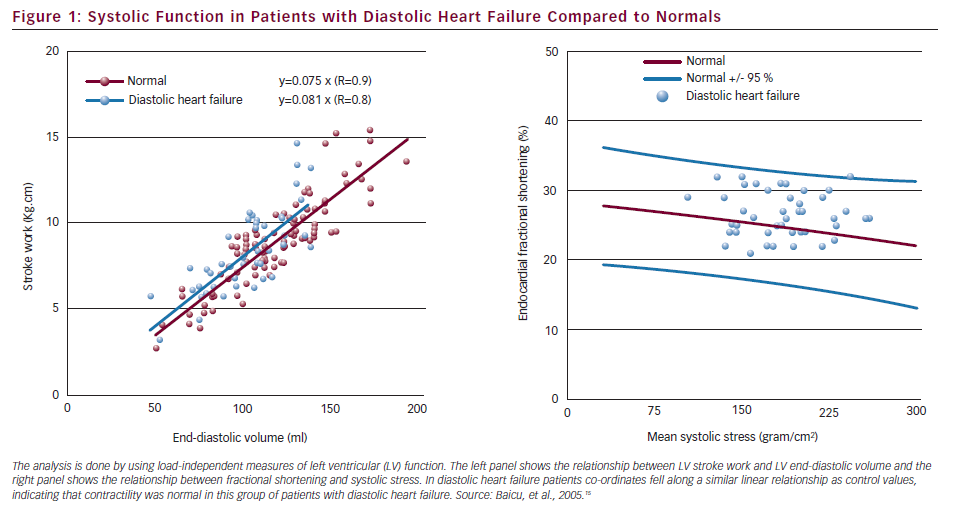
There has been some controversy about the frequency of HF-PEF, and due to the relatively nonspecific nature of heart failure symptoms, it has even been questioned if the symptoms may have alternative explanations, such as obesity and lung disease.13 This underscores the importance of searching for objective signs of diastolic dysfunction. Furthermore, because EF is a relatively crude measure of systolic function, it has been debated whether systolic function is completely normal in patients with HF and normal EF. The observations that LV long axis shortening may be reduced during resting conditions,9 and attenuated increase in stroke volume during physical exercise14 support the view that there is a problem with systolic function. There is reason to believe, however, that HF-PEF represents a spectrum of disturbances of LV function, spanning from patients with mildly reduced systolic function to those with essentially normal contractility. Caution should be exerted, however, when interpreting indices of myocardial fiber shortening such as myocardial velocity, mitral annular velocity, atrio-ventricular plane displacement, or myocardial strain, which are all load-dependent since a reduction in any of these indices may reflect increased afterload rather than reduction in contractility. Furthermore, reduced long-axis shortening may be due to increased chamber stiffness, which leads to reduced diastolic filling with reduced utilisation of the Frank–Starling mechanism. Therefore, reduced long-axis shortening in HF-PEF patients may not in every case reflect systolic dysfunction. In an attempt to resolve this issue Baicu et al.15 studied a group of patients with HF-PEF and quantified LV systolic function by methods which accounted for loading conditions. They concluded that in their group of HF-PEF patients, LV systolic function and contractility were normal (see Figure 1). Therefore, there appears to be a group of patients with essentially isolated diastolic heart failure, and this might include patients with LV hypertrophy secondary to arterial hypertension.
As most patients with systolic heart failure have impairment of diastolic function, systolic and diastolic heart failure should not be considered as entirely different disease entities. In diastolic heart failure, however, the pathophysiology is dominated by abnormal LV filling properties, while loss of contractile force dominates in systolic heart failure.
Patients with systolic and diastolic heart failure have similar symptoms and signs, and therefore clinical history and physical examination do not differentiate between the two conditions. For this reason and because the heart failure diagnosis is based on relatively non-specific symptoms and signs, it is important to incorporate an objective measure into the set of diagnostic criteria. Therefore, in keeping with the recommendations from the European Study Group on Diastolic Heart Failure7 we recommend that the diagnosis of diastolic heart failure is restricted to patients in whom there is either invasive or non-invasive evidence of diastolic dysfunction.
Aetiology of Heart Failure with Preserved Ejection Fraction
Diastolic heart failure or HF-PEF is not a specific disease, and it is therefore important to search for underlying disorders. The potential aetiologies include arterial hypertension, coronary artery disease, restrictive cardiomyopathy, cardiac amyloidosis, and hypertrophic cardiomyopathy. When HF-PEF occurs in patients with systolic hypertension, it is associated with LV hypertrophy, often in combination with coronary artery disease and/or diabetes.1,16 Each of the conditions are associated with variable degrees of structural remodelling and stiffening of the cardiovascular system. In hypertension there is thickening and deposition of collagen in the arterial walls, which leads to stiffening of the arterial system. This implies increased afterload for the left ventricle, which serves as a stimulus to LV remodelling, resulting in concentric hypertrophy and increased LV diastolic stiffness. As suggested by animal studies, the increased LV stiffness is due to a combination of increased LV wall thickness and myocardial fibrosis.17 The latter change appears to be due to activation of the renin–angiotensin–aldosterone system and different neurohormones.17 In addition to the structural changes, arterial stiffening in hypertension leads to higher systolic LV wall stress, which results in slowing of LV relaxation.18
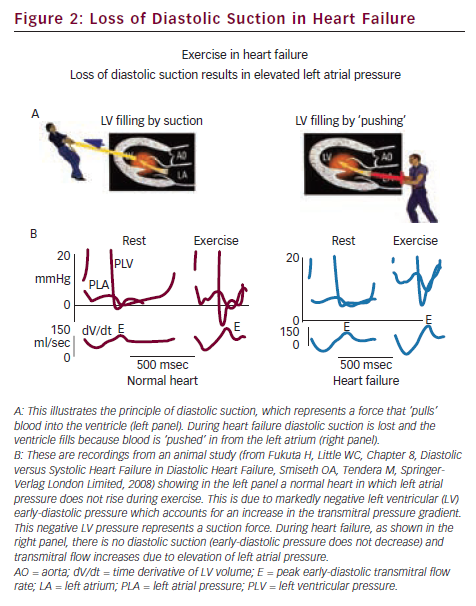
The underlying cardiac dysfunction in diastolic heart failure is slowing of LV relaxation and increased LV chamber stiffness, which leads to elevated LV diastolic pressure. The problems are aggravated during physical exercise, when compensatory mechanisms cause further elevation of LV end-diastolic pressure in an attempt to distend the stiff ventricle and thereby increase stroke volume by the Frank–Starling mechanism.19 The resulting pulmonary congestion due to elevated LV diastolic pressure and the limited increase in stroke volume due to a stiff ventricle explain the exertional dyspnoea and fatigue in diastolic heart failure. This is in contrast to healthy individuals with a compliant ventricle and compliant arteries, who can increase cardiac output during exercise by minimal increase in filling pressure.19 This principle is illustrated in Figure 2, which shows that in the normal heart transmitral flow is increased during exercise by an increase in the transmitral pressure gradient with little or no rise in left atrial pressure. This is explained by a marked lowering of LV early diastolic pressure to negative values and represents a suction force which ‘pulls’ blood into the ventricle. Therefore, increased filling during exercise in a normal heart is explained by diastolic suction. During heart failure, however, there is typically a slowing of relaxation and reduction of restoring forces, which lead to elevated early-diastolic pressure and loss of diastolic suction. Since the failing heart has lost its ability to lower LV early-diastolic pressure, the only way to increase the transmitral pressure gradient and thereby LV filling during exercise, is by an increase in left atrial pressure. This mechanism explains the elevation of pulmonary capillary pressure during exercise in patients with heart failure and the mechanisms are in principle similar in systolic and diastolic heart failure.
A consequence of the stiff cardiovascular system that may be found in patients with HF-PEF, is that a small increase in blood volume as after meals with high salt content, can move the patients into overt heart failure. Similarly, aggravation of hypertension which will elevate LV diastolic pressure, can precipitate flash (rapid-onset) pulmonary oedema.20 Taken together, these observations suggest that stiffening of the arteries and elevated afterload, which causes LV remodelling, may be an important mechanism in the development of HF-PEF. More work is needed, however, to substantiate this as a general mechanism.
It has been observed that a significant portion of HF-PEF subjects have chronotropic incompetence, as indicated by blunted heart rate response to exercise, and it is likely that this mechanism contributes to their reduced exercise tolerance.21,22
Normal ageing is also associated with arterial stiffening and is clinically evident as an age-dependent gradual rise in systolic pressure and in arterial pulse pressure.23 This is due to vascular remodelling, which includes thickening of the arterial walls, particularly the intima, and increasing amount of collagen in the media. Therefore, the ageing process further augments the problems that are due to hypertension.
Ageing is associated with a shift in LV filling from early to late diastole. This is reflected in transmitral flow velocities as a decrease in early-diastolic velocity (E) and an increase in atrial-induced velocity (A), with reversal of the E/A velocity ratio. Similarly, LV lengthening velocities by tissue Doppler of the mitral annulus demonstrate an age-dependent decrease in peak early-diastolic lengthening velocity (e’), and an increase in atrial-induced lengthening velocity (a’) with ageing. Therefore, elderly people often have a filling pattern that is typical for patients with impaired LV relaxation, as can be seen in the early stages of many cardiac diseases and in LV hypertrophy. It is important, however, not to mix up diastolic dysfunction and diastolic heart failure, and it is not known if the age-related diastolic dysfunction identifies patients who are at increased risk of developing heart failure.
Systolic function in the healthy elderly appears normal since EF is similar to that in younger individuals. In the healthy elderly, however, there is reduced contribution from longitudinal shortening, which is balanced by an increase in circumferential shortening.24 This shift may contribute to the age-dependent reduction in e’.
How to Diagnose Heart Failure with Preserved Ejection Fraction
The heart failure diagnosis is based on history, clinical examination and on objective measures of cardiac dysfunction. Symptoms and signs include dyspnoea, tachypnoea, cough and abnormal auscultatory findings over the heart and lungs. There may also be secondary right-sided heart failure with distended neck veins and other signs of elevated central venous pressure. The two haemodynamic hallmarks of diastolic dysfunction are impaired LV relaxation and increased diastolic stiffness, which lead to compensatory elevation of LV filling pressure.
Consistent with the consensus reports from the European Study Group on Diastolic Heart Failure7 we recommend that the diagnosis of diastolic heart failure is restricted to patients in whom there is objective evidence which supports the diagnosis. Since invasive studies are rarely available, the objective diagnostic evidence is most often limited to non-invasive data. The most important diagnostic information comes from echocardiography and includes cardiac functional as well as structural data. Natriuretic peptides provide additional diagnostic information, but have a limited role.
Invasive Methods
Measurement of Left Ventricular Filling Pressure
The term LV filling pressure is used for both left atrial mean pressure and LV end-diastolic pressure. There is often a slight difference between the two pressures, but this is rarely of clinical significance as long as there is no mitral stenosis. Left atrial pressure is a more direct determinant of pulmonary capillary pressure, while LV end-diastolic pressure is the best measure of preload when studying LV mechanical function. In rare cases left atrial pressure and LV end-diastolic pressure do not reflect preload, as in cardiac tamponade and during mechanical ventilation with positive end-expiratory pressure, when LV end-diastolic pressure is elevated due to increased pressure external to the ventricle. In general, however, both left atrial pressure and LV end-diastolic pressure are excellent measures of LV preload, and for clinical purposes they can be used interchangeably. Strictly speaking, LV preload is best measured as LV transmural pressure, which equals LV end-diastolic pressure minus pericardial pressure. This is feasible during invasive studies when using mean right atrial pressure as an estimate of pericardial pressure.25,26
In patients undergoing left heart catheterisation, LV filling pressure can be measured directly as LV end-diastolic pressure, and during right heart catheterisation as pulmonary capillary wedge pressure (PCWP). The latter is an indirect, and in most cases accurate measure of mean left atrial pressure. Alternatively, one may use pulmonary artery diastolic pressure as an estimate of mean left atrial pressure, provided there is normal pulmonary circulation and no significant mitral regurgitation.
Measurement of Left Ventricular Relaxation and Diastolic Stiffness
Global LV relaxation can be quantified by measuring how rapidly LV pressure falls during isovolumic relaxation. This is measured as the time constant of LV isovolumic pressure fall (tau). When tau is prolonged, it indicates slowing of global LV relaxation. Measurement of tau requires high-sensitivity catheters, and can not be done with the fluid-filled catheters which are used during routine left heart catheterisation. The added diagnostic value of measuring tau has not been fully explored. Therefore, invasive assessment of LV relaxation is rarely justified in clinical routine.
Evaluation of LV stiffness by analysing pressure-volume curves is done as part of research protocols, but has no role in clinical routine.
Non-invasive Methods
Evaluation of Left Ventricular Filling Patterns
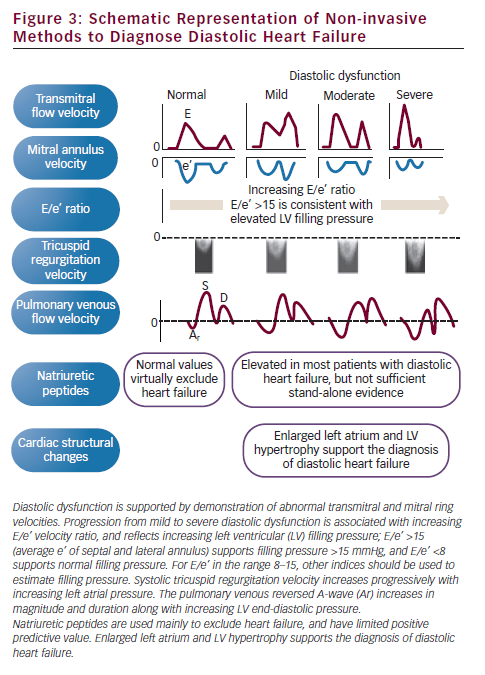
In the early stages of diastolic dysfunction there is typically slowing of LV relaxation, which causes a decrease in peak E-velocity and a compensatory increase in peak A-velocity.27,28 This shift in filling from early to late diastole, measured as a decrease in the E/A ratio, is described as a pattern of impaired relaxation. As heart failure progresses and left atrial pressure becomes elevated, there is an increase of the early-diastolic transmitral pressure gradient, which increases peak E-velocity, and the E/A ratio may become normal. This is described as a pseudonormalised filling pattern. When heart failure progresses further, left atrial pressure may become markedly elevated and the early-diastolic transmitral pressure gradient increases, leading to supernormal peak E-velocity. Since elevation of LV diastolic pressure causes the ventricle to operate on a steeper portion of its pressure-volume curve, indicating reduced chamber compliance, there is little further increase in LV volume during atrial contraction. This is measured as a small and abbreviated transmitral A-velocity. Furthermore, due to reduced LV chamber compliance, the early transmitral flow decelerates rapidly. This filling pattern with increased E/A ratio and short E-deceleration time is described as restrictive physiology. An additional feature of this filling pattern is abbreviated isovolumic relaxation time (IVRT) due to premature opening of the mitral valve caused by the elevated left atrial pressure. Since assessment of mitral flow velocities alone does not differentiate between pseudonormal and true normal filling, there is need for an additional technique. The best and easiest additional method is measurement of peak early-diastolic mitral annulus velocity (e’), which is reduced in patients with pseudonormalised filling. In patients with impaired relaxation and restrictive physiology there is also reduced e’ (see Figure 3).
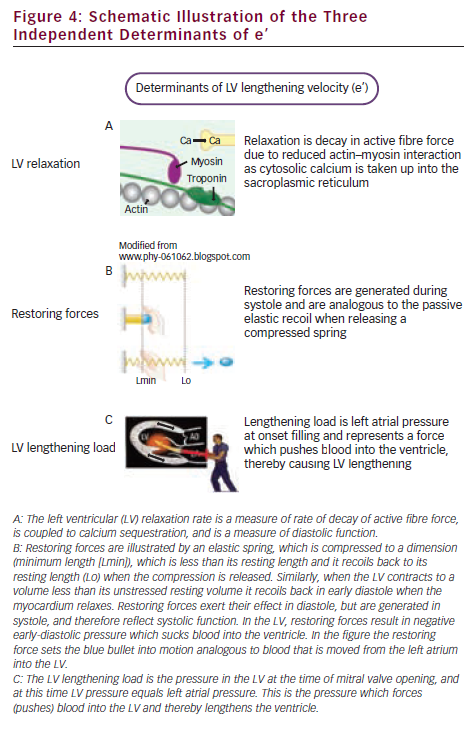
The early-diastolic mitral annulus velocity is a measure of LV lengthening velocity and is determined not only by diastolic function. As shown by Opdahl et al.,29 peak e’ is determined by rate of LV relaxation, by LV restoring forces and by LV diastolic load (lengthening load). These determinants are illustrated and explained in Figure 4.
Estimation of Left Ventricular Filling Pressure
A number of echocardiographic indices may be utilised to estimate LV end-diastolic or left atrial mean pressure and these methods are presented in a consensus document from the European Association of Echocardiography jointly with the American Society of Echocardiography.30 In patients with heart failure and reduced EF the echocardiographic indices provide accurate diagnostic information which can be used to identify patients with elevated filling pressure. In patients with normal EF, however, the use of these indices is more challenging. The E/e’ ratio, however, represents one of several approaches which may be of clinical value. It is recommended to use the average e’ of septal and lateral mitral annulus recorded in an apical view, and a ratio <8 identifies patients with normal LV filling pressures, whereas an average ratio >15 indicates an increase in LV filling pressures. When the ratio is between eight and 15, other measurements are essential. It is important to be aware that the values for E/e’ ratios are based on e’ recorded by pulsed tissue Doppler imaging (TDI), which gives peak velocities. This is in contrast to e’ by 2D colour mode which provides mean velocities and are approximately 20–30 % lower than peak velocities.
Additional indices that are clinically useful include reversed pulmonary venous velocity and peak tricuspid regurgitation velocity. One advantage of considering the E/e’ ratio, peak tricuspid regurgitation velocity and reversed pulmonary venous flow during atrial contraction is that the effects of normal ageing appear to be eliminated, and these indices become more reliable markers of elevated filling pressure. Figure 3 shows schematically relevant echocardiographic measures, which may be of value when evaluating patients with HF-PEF. It is important to look for consistency between indices and not trust only one measure.
Provided signal quality is good, which is the case in approximately 25–30 % of patients with transthoracic imaging, the pulmonary venous reversed velocity during atrial contraction provides reliable information. If the duration of retrograde pulmonary venous flow exceeds antegrade transmitral flow with >30 ms, it is very likely that LV filling pressure is elevated.30,31 Furthermore, the peak value of reversed flow velocity increases along with elevation in LV filling pressure. The method has some limitations, including reduced atrial systolic function. Estimation of pulmonary artery systolic pressure from peak tricuspid regurgitation velocity can be achieved in most patients and is part of almost every routine echocardiographic examination. Important limitations to this approach are lung disease and severe mitral regurgitation, but both these conditions can usually be sorted out. Systolic pulmonary artery pressure is estimated as the sum of the estimated right atrial pressure and the systolic tricuspidal pressure gradient. Provided there is no pulmonary vascular disease, an elevated pulmonary artery pressure is most likely a sign of elevated left atrial pressure.
Diastolic Stress-echocardiography
Patients with HF-PEF often have breathlessness during exercise, which is related to elevation of the LV end-diastolic pressure without an accompanying increase in end-diastolic volume.19 The physiological increase in stroke volume is therefore reduced or absent. It has been demonstrated that E/e’ correlates with LV end-diastolic pressure (LVEDP) during exercise testing of patients with HF-PEF.32 Although further validation is needed, a cut-off value of exercise E/e’ of 13 is suggested to identify patients with diastolic heart failure.33 It is noteworthy that, as dobutamine has other haemodynamic effects than exercise testing, there is no significant correlation between E/e’ and LVEDP during dobutamine testing.34
Magnetic Resonance Imaging/Nuclear Methods/Computed Tomography
Neither magnetic resonance imaging (MRI), radionuclide-based methods nor computed tomography (CT) has a role in routine evaluation of patients with diastolic heart failure. There are exceptions such as constrictive pericarditis or pure myocardial diseases, which require more comprehensive imaging.
Structural Changes
Increased left atrial volume may also be used as objective evidence of diastolic function. Furthermore, LV hypertrophy is consistent with impaired diastolic function and supports the diagnosis.
Blood Markers
Elevated B-type natriuretic peptide (BNP) or N-terminal pro-BNP (NT-proBNP) may be used to support the diagnosis diastolic heart failure, but is not considered sufficient stand-alone evidence for diastolic dysfunction.35,36 Natriuretic peptides are recommended mainly for the exclusion of the diagnosis, which is justified by its high negative predictive value for heart failure.37
Differential Diagnosis
Since symptoms and signs of diastolic heart failure are relatively non-specific, it is important to exclude non-cardiac aetiologies, in particular lung disease. It is also important to exclude other cardiac disorders, such as valvular heart disease and coronary artery disease. Valvular heart disease is a well-known cause of heart failure and is easily identified by echocardiography. Symptoms of coronary artery stenosis can mimic those of heart failure, in particular in diabetic patients. Therefore, a stress test or coronary angiography should be considered. In summary, a diagnostic work-up for patients with suspected diastolic heart failure can be done at low cost in almost every cardiology practice. When invasive data are not available, echocardiography is the preferred method to determine if there is LV diastolic dysfunction. In general practice, blood markers such as BNP may be used as the initial method, and when BNP is normal, it is very unlikely that the patient has any form of heart failure. Importantly, this does not mean that the patient has no heart disease, and it may be required to search for valve disease, coronary artery disease or other cardiac disorders. When blood markers are elevated, however, heart failure is likely and the patient should be referred for echocardiography to define underlying pathology and to confirm the diagnosis.
Prognosis and Treatment
Patients with HF-PEF have a prognosis which is almost as severe as for patients with heart failure and reduced ejection fraction.5,38 The poor prognosis may in part reflect an effect of co-morbidities.
Effective treatment of diastolic heart failure has not yet been established, but ongoing clinical trials may provide some answers. Due to the limited documentation it is difficult to give firm recommendations. At the present stage it seems reasonable to use symptomatic treatment with similar drugs as in heart failure with reduced systolic function. For patients with hypertension, however, lowering of blood pressure results in an approximately 50 % reduction in the risk of developing heart failure.39,40 These studies demonstrate that prevention of diastolic heart failure by treating arterial hypertension is effective therapy and is currently the best documented therapeutic approach.