Heart-brain interaction is an indisputable fact. The connection between vascular risk factors and/or cardiovascular disease and cognitive impairment and dementia has been supported by countless publications in the past 30 years. The most important studies concluded that vascular brain injury exacerbates cognitive ageing, and increases the risk for dementia both in its vascular type and in its degenerative type (Alzheimer’s disease). However, the negative impact of vascular risk factors depends on, first, the length of exposure to vascular injury, and second, the burden of vascular injury accumulated over time.
This connection, complex in essence, is actually an inverse problem because the aim is to identify the past circumstances that have led to heart and/or brain disease. A myriad of strategies for controlling the problem may arise from the interpretation of the facts and the plausible associations derived from scientific observation.
Over the past 50 years, vascular medicine – based on major technological developments – has significantly extended lifespan, but has failed to put forth prevention strategies to reduce heart and cerebrovascular morbimortality. Timely intervention on modifiable vascular risk factors might diminish the prevalence of both cardiovascular and neurocognitive diseases, and place prevention at the core of the matter. A deep understanding of the heart-brain interaction will enable the implementation of health policies that ensure normal brain ageing, and thus longer preservation of intellectual capacity and autonomy. This review intends to discuss this heart-brain connection, describe how uncontrolled vascular disease impacts on cognition, and emphasise the importance of prevention and health promotion messages.
This review is based on bibliographic consultations in: MEDLINE, SciELO, EMBASE, LILACS, and other data sources. The terms employed for the search were: cognitive impairment, dementia, Alzheimer’s disease, vascular risk factors, and cardiovascular prevention. Bibliography from the past 10 years was examined including review studies, original research papers, population-based epidemiological studies, and intervention studies.
Vascular Risk in Cognitive Diseases
In 2010, the joint report of the World Health Organisation and Alzheimer’s Disease International claimed that there were 35.6 million people with dementia in the world, and anticipated that the figure would almost triple by 2050 (115 million)1. Four years later, the projection was increased by 15 % (135 million people with dementia)2. Longer life expectancy has contributed to this epidemic growth, especially in the population aged 60 or over, and particularly in the over-80 age group. According to the World Health Organisation, in high-income countries, average life expectancy is 8.7 years for 80-year-old men and 11 years for 80-year-old women, (30 % more than three decades ago)3. The main reason for this occurrence is the continuing drop in mortality rate from heart and cerebrovascular diseases due to the technological advances of the past 50 years. Identification of vascular risk factors, new drugs that have been developed and new techniques, such as coronary artery bypass grafting, balloon angioplasty and stent implants, paradoxically seem to have contributed to the main risk factor for dementia in old age. Medicine has improved the prognosis of manifest vascular disease, and it has also prolonged life expectancy; however, heart and cerebrovascular diseases are still the main cause of death throughout the world (≈30 %)4.
The same vascular risk factors that affect cardiovascular health also compromise cerebrovascular health. Vascular brain injury and the resulting cellular damage (oxidative stress, swelling) appear to be the causes of the altered brain ageing process, leading to increased risk for stroke, cognitive decline, dementia, depression, and other neurological problems, such as gait disorders.
From this perspective, the prevalence of dementia will continue to increase as long as the scientific method is systematically focused on the treatment for the dementia syndrome and not on its prevention. The only way to reduce or eradicate its incidence is by implementation of preventative approaches. In accordance with the World Health Organisation definition, prevention covers measures which not only to prevent the occurrence of disease, but also arrest its progress and reduce its effects once established, thus, it is imperative to appraise population vulnerability; i.e. ascertain what it is we have to prevent.
Cardiovascular Health and Incidence of Dementia Decline
Despite the fact that dementia has reached epidemic levels due to the increasing number of people that are affected by it, recent publications have reported its incidence is actually declining. This trend may follow improvements in education quality and more effective control of vascular risk factors.
Both the Mayo Clinic Study (2005)5 and the Health and Retirement Study (2008)6 informed a 50 % drop in the prevalence of cognitive decline (from 5.7 % to 2.9 %) in a 17-year period, and a 29 % drop (from 12.2 % to 8.7 %) in a nine-year period. The retrospective interpretation of these results is that this decrease stems from a reduction of the stroke rate, improvements in health education and favourable changes in lifestyle (i.e. control of vascular risk factors).
Three European studies (Rotterdam Study, Cognitive Function and Ageing Study I-II and a study carried out in the Swedish population)7,8,9, with similar results, agree that the explanation may be better control of vascular disease and vascular risk factors.
The comparison of two sub-cohorts in the Rotterdam study (10 years apart) underscored the finding that in the most recent cohort, the incidence of dementia was lower, the participants’ brains were bigger (i.e. less atrophy) with fewer white matter lesions7 , and this was attributed to the fact that this group took more anti- hypertensive and antithrombotic (aspirin) drugs and statins.
In a nutshell, “…there is evidence from various studies that in high- income countries, the incidence of dementia is decreasing due to high levels of education and improvement in cardiovascular health.” (Alzheimer’s Report 2014)2.
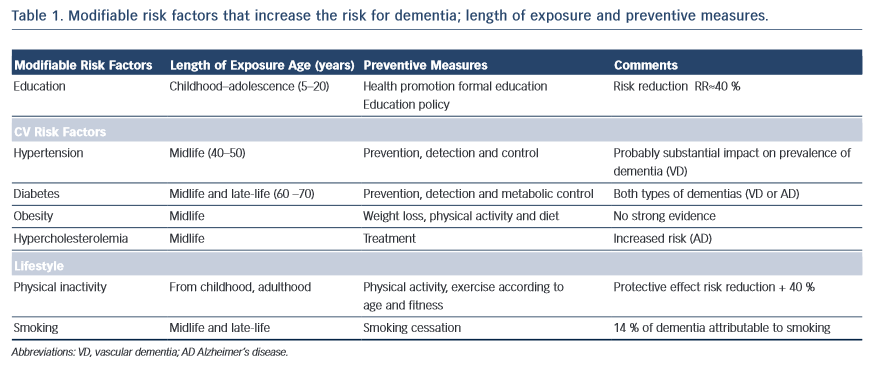
In essence, every case of vascular disease will be somehow associated with vascular brain injury and, consequently, with neurocognitive diseases. Middle-age hypertension is related to the progression of white matter lesions, cognitive impairment and mood disorders10,11, diabetes seems to add loss of brain volume to vascular lesions, and obesity has also been associated with higher risk for dementia13. Cognitive impairment in patients with metabolic syndrome appears to be directly linked to the number of components encompassed in the particular syndrome and the metabolic disorder involved14; smoking more than two packs of cigarettes a day increases the risk for dementia 20 years later15, and atrial fibrillation with inadequate anticoagulation therapy increases the risk for stroke and dementia (Alzheimer’s disease) irrespective of vascular disease16,17 (see Table 1).
New studies have reported that aggregate exposure to vascular risk factors since early stages in life is also associated with worsening cognition in mid-life18. Accordingly, identification of vascular risk factors and the duration of exposure when they may have had a negative impact on health can be considered targets for prevention.
Physiopathological Heart – Brain Connection
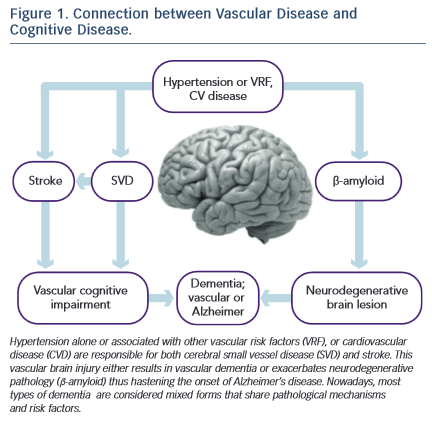
There are three disorders through which vascular disease brings on mild cognitive decline or dementia: i) stroke, ii) small vessel disease, and iii) -amyloid depositions (A ) that contribute to neurodegenerative phenomena. (see Figure 1)
Stroke
Fifty per cent of strokes may be attributed to hypertension, and they constitute the main risk factor for cognitive impairment or post-stroke dementia. The prevalence of post-stroke cognitive decline has a broad range, from 20 % to 80 %19. This variability depends on the diagnostic tools and the criteria employed, on the extent of the vascular and neurodegenerative pathologies before the stroke, on the cognitive status, on the stroke extension (volume) and topography (i.e. strategic areas such as frontal cortex, hippocampus or white matter).
Cerebral blood flow self-regulation alterations induced by brain ageing, vulnerability to hypoperfusion and hypoxia, lacunar infarcts (responsible for 70–90 % of vascular cognitive impairment), micro-haemorrhages, and neuron damage (protein synthesis and synaptic plasticity) account for the complex association between stroke and cognition.
Post-stroke cognitive status is not confined to vascular cognitive impairment or vascular dementia; it may also play a role in the genesis of Alzheimer’s disease and mood disorders (depression).
The Nun’s Study has shown that lacunar infarcts cause a 20-fold increase in the risk for Alzheimer’s disease20, and the PROGRESS Study (Perindopril Protection against Recurrent Stroke Study) has indicated that hypertension treatment reduces the risk for post- stroke dementia in 34 %21. Depression in older adults is closely related to subcortical vascular pathology, thus supporting the vascular depression hypothesis22. This syndrome presents a high post-stroke prevalence that ranges from 35 % to 50 %.
Small Vessel Disease
Small vessel disease is essentially associated with hypertension. The blood vessels supplying the white matter (penetrating arteries, branches of subarachnoid and subependymal arteries) are terminal arteries. The absence of collateral circulation renders the periventricular regions vulnerable to atherosclerotic ischaemic damage. The ischaemic episodes and the reactive gliosis lead to a process called araiosis (rarefaction) that damages the nerve myelin.
White matter hyperintensities (leucoaraiosis) and lacunar infarcts seen in neuroimages are the objective signs of these vascular abnormalities that cause white matter demyelination and subcortical-cortical disconnection or desaferentisation syndrome. The disconnection of the dorsolateral prefrontal cortex – the most affected circuit –gives rise to a cognitive syndrome called executive dysfunction, a distinctive feature of vascular brain injury and commonly seen in hypertensive patients23,24. On the other hand, selective neocortical atrophy (grey matter volume in the frontal lobes) may also contribute to the executive dysfunction25.
Our group has shown that patients with hypertension have a five-fold increased risk for executive dysfunction in the course of the disease26. Early detection of this syndrome is important because it increases the risk for conversion to dementia27 and/or to major depression22.
White matter disease, microinfarctions and micro-bleedings may have an impact on the onset of cognitive dysfunction. The volume or burden of white matter lesions, along with their progression and their location determine the clinical expression of the cognitive impairment and increase the risk for stroke. Limits to progression and burden depend on effective blood pressure control28.
-amyloid depositions
-amyloid is a protein that accumulates in brain extracellular spaces (neuritic plaques) and on the blood vessel walls (cerebral amyloid angiopathy). It results from the abnormal cleavage of a transmembrane precursor protein and constitutes the neuropathological basis of Alzheimer’s disease29, along with the neurofibrillary tangles (protein aggregates of hyperphosphorylated tau protein). The vascular damage observed in almost 90 % of the brains of patients with Alzheimer’s disease contributes to the progress of the neurodegenerative process which, in turn, leads to its clinical expression (dementia), supporting the hypothesis that vascular and neurodegenerative injuries are the extreme points of a spectrum, within which most of the various forms of dementia can be found.
Neurodegenerative pathology seems to be a necessary but not sufficient condition. The vascular damage (endothelial dysfunction, ischaemia, blood-brain barrier disruption) alters the balance between A production and clearance, thus increasing A levels in the brain with the toxic consequences (reactive oxygen species production and inflammatory response)30.
Recent publications report that patients with treated but uncontrolled hypertension increase their brain A depositions31,32,33. Knowledge of the role vascular injury plays in the clinical expression of Alzheimer’s disease may open up new avenues to explore in the search for dementia prevention by employing strategies that either treat or prevent the vascular cause.
Vascular Prevention of Cognitive Diseases
For more than two decades, vascular disease has been gaining ground within neurodegenerative diseases, among which dementias have a significant place. Risk factors that impair vascular health – hypertension, hypercholesterolaemia and diabetes – contribute to hastening and compounding dementia and worsen its prognosis.
Considering the above hypotheses and published research devoted to investigating the link between vascular disease, cognitive decline and dementia, we can conclude that: i) patients with hypertension, whether isolated or associated with other vascular risk factors, present vascular brain injury and greater risk for dementia than the vascular disease- free population; ii) cases of Alzheimer’s disease are paradoxically more frequent than cases of vascular dementia among patients with vascular risk factors and vascular disease; iii) the association of vascular brain injury and brain neurodegenerative disorder is clinically expressed by greater cognitive decline; and iv) some studies have proved that the preservation and enhancement of vascular health through rigorous control of risk factors may prevent or delay the onset of dementia, and in those already diagnosed, may contribute to slowing of cognitive decline. Therefore, the promotion of vascular health becomes the focal point of primary and secondary prevention of dementias34.
Today we can conclude that vascular risk factors are shared by heart and brain. As a result, if we deem the seventh decade of life as the average age in which late-onset Alzheimer’s disease begins, our preventive attitude should target vascular risk factors 20 years earlier, that is, the mean age in which these factors’ presence becomes relevant. Furthermore, a recent publication points out that more than 30 % of late-onset Alzheimer’s disease cases may be attributed to the combination of vascular risk factors35.
Thus, if timely intervention manages to increase the control of vascular risk factors in the vulnerable population by 10 %, the projected effect would be a reduction in the number of cases of Alzheimer’s disease by one million, and by three million if control reaches 25 %. In other words, if we could delay onset of Alzheimer’s disease by five years, cases of dementia would be reduced by half within 10 years36.
In 2011, the Alzheimer’s Disease International World Report recommended antihypertensive drugs, statins and physical activity among the most beneficial interventions for dementia prevention. All of these measures are aimed at preserving vascular health37, and do not exclude the need to recognise, reduce and treat vascular risk factors or cardiovascular disease in patients with dementia (see Table 1).
Prevention should include health promotion to inspire a change in the population towards healthier lifestyles and behaviours, while also raising awareness among physicians about these factors. In this way, the epidemic levels of dementia currently seen will be effectively managed by a population controlling its own health and through the identification of vulnerable individuals by the healthcare sector.
Some of the variables that operate in the clinical expression of dementia escape intervention (e.g. genetics, age), while others are modifiable. However, vascular risk factors that impact negatively on vascular health can be controlled. We cannot limit our efforts and simply wait until a drug treatment that can cure Alzheimer’s disease makes its appearance on the market (although that drug is necessary). Instead, our purpose should be to highlight prevention and promote vascular health as the only effective method for reducing the prevalence of Alzheimer’s disease.
Summary and Final Comments
Dementia is, as yet, an incurable disease. There is evidence however to support the idea that targeting the vascular component of the disease with preventative measures may delay the onset of the condition. Translational research involving relevant medical specialties is essential to solving the difficult problem posed by dementia. Vascular risk factor detection, preventative measures and the identification of therapeutic targets during the early stages of life may represent an effective strategy to prevent or delay the onset of cognitive decline and dementia later on. Further research is required to understand the molecular mechanisms of the disease and this will provide the basis for the development of rational treatments.