Coronary artery disease (CAD) due to atherosclerosis is a major cause of morbidity and mortality. Early prevention of atherothrombotic disease with a healthy lifestyle (diet, exercise, optimal body weight and no smoking) is considered the best method of “treating” CAD, although increasing age remains associated with significant cardiovascular events.
When coronary atherothrombotic disease becomes clinically significant, it can evolve into stable ischaemic heart disease or an acute coronary syndrome (ACS). In ST-elevation ACS, an invasive approach with early coronary angiography and revascularisation of the culprit lesions can reduce mortality and promptly alleviate symptoms. Recent studies advocate early complete revascularisation of all coronary stenoses greater than 50 % in ACS, although this still remains a matter of debate.1 It is presumed that in unstable clinical conditions full revascularisation is warranted, irrespective of the real ischaemic significance of a coronary stenosis.2
In stable CAD revascularisation is a much more debatable issue due to the lack its effect on the hard endpoints of unselective intervention based only on the angiographic severity of a coronary stenosis. Revascularisation should be reserved for patients who have both coronary artery stenoses at angiography and myocardial ischaemia due to significant flow reduction.
This review supports the use of objective proof of inducible myocardial ischaemia prior to deciding whether a patient with stable CAD and moderate stenoses should undergo revascularisation.
Identification of Atherosclerotic Coronary Lesions on Angiography
Current indications for coronary angiography in patients with stable ischaemic heart disease are limited to high-risk clinical conditions determined by non-invasive testing. Some patient subsets have a mortality risk greater than 3 % per year.3,4 Patients who have survived an episode of sudden cardiac arrest, have high-risk spontaneous ventricular arrhythmias, who develop unexplained heart failure or who have high-risk criteria at non-invasive stress testing should undergo coronary angiography3,4 as it allows the assessment of coronary anatomy and may be the deciding factor in whether a patient should undergo revascularisation.
It is currently accepted that revascularisation by percutaneous coronary intervention (PCI) or coronary artery bypass graft (CABG) should be performed when: a stenosis >50 % is observed during angiography of the left main (LM) stem or proximal left anterior descending (LAD) coronary artery; the patient has a single remaining patent vessel; or suffers from two- or three-vessel disease with a left ventricular ejection fraction <40 %.5 Another indication for revascularisation resulted from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial: any coronary stenosis >50 % that can produce myocardial ischaemia in >10 % of the left ventricular myocardium.5,6
Many lesions of the coronary arteries fall outside these clearcut situations. Some patients have critical coronary stenoses in vessels that have no distal run-off or irrigate non-viable myocardium.
Other patients have moderate coronary lesions with between 50 and 70 % stenosis with no clinical proof of myocardial ischaemia, and the decision whether or not to revascularise them is made by the interventional cardiologist based on this “occulo-stenotic reflex”. Others have diffuse plaque burden and negative remodelling with no critical stenoses, but have significant inducible myocardial ischaemia. A recent review of all randomised trials in this patient category demonstrated that the worst prognosis was observed in patients with both focal stenoses and global diffuse disease, followed by those with diffuse disease and no focal stenoses; patients with focal stenoses, but no diffuse disease had also better long-term outcomes.7
Currently 20–30 % of all coronary revascularisation procedures are performed in patients with stable CAD, raising the issue of how appropriate these procedures are in individuals with moderate coronary artery stenosis.8 Revascularisation of the “intermediate coronary lesions” with stenoses of between 50 and 70 % should be based on accurate physiological measurements of impaired epicardial blood flow obtained in the catheterisation laboratory (cath-lab) by measures of intracoronary pressure (the fractional flow reserve, FFR) or coronary flow (coronary flow reserve, CFR) by intracoronary Doppler.9 Recent advances in non-invasive angio-computed tomography (CT) allow indirect assessment of FFR by computation from static coronary CT images using computational fluid dynamics (CT-derived computed FFR or FFRCT).10,11 Multiple clinical trials have proven the value of the physiological assessment of coronary atherosclerosis determined either by FFR or non-invasive CFR for assessing prognosis in stable CAD.12,13
Physiologically Assessing Coronary Stenosis in the Catheterisation Laboratory
Intermediate coronary stenoses of between 50 and 70 % induce myocardial ischaemia in 35 % of cases; of lesions with 71–90 % stenoses, some 80 % are functionally significant; and lesions with between 91 and 99 % stenoses induce myocardial ischaemia in 96 % of cases.14 Appropriate identification of ischaemia-inducing lesions is thus mandatory.
FFR is the ratio between pressure distal to a coronary stenosis and aortic pressure at maximum vasodilatation induced by a potent coronary vasodilator, usually adenosine.15 Different doses are given in the left and right coronary arteries (100–200 μg and 50–100 μg, respectively); a continuous intravenous adenosine infusion may be used instead of direct intracoronary administration at a rate of 140 μg/kg/min. Alternative agents include intracoronary nitroprusside, papaverine or nicorandil and intravenous regadenoson (an A2A receptor blocker), but the standard FFR drug is adenosine.
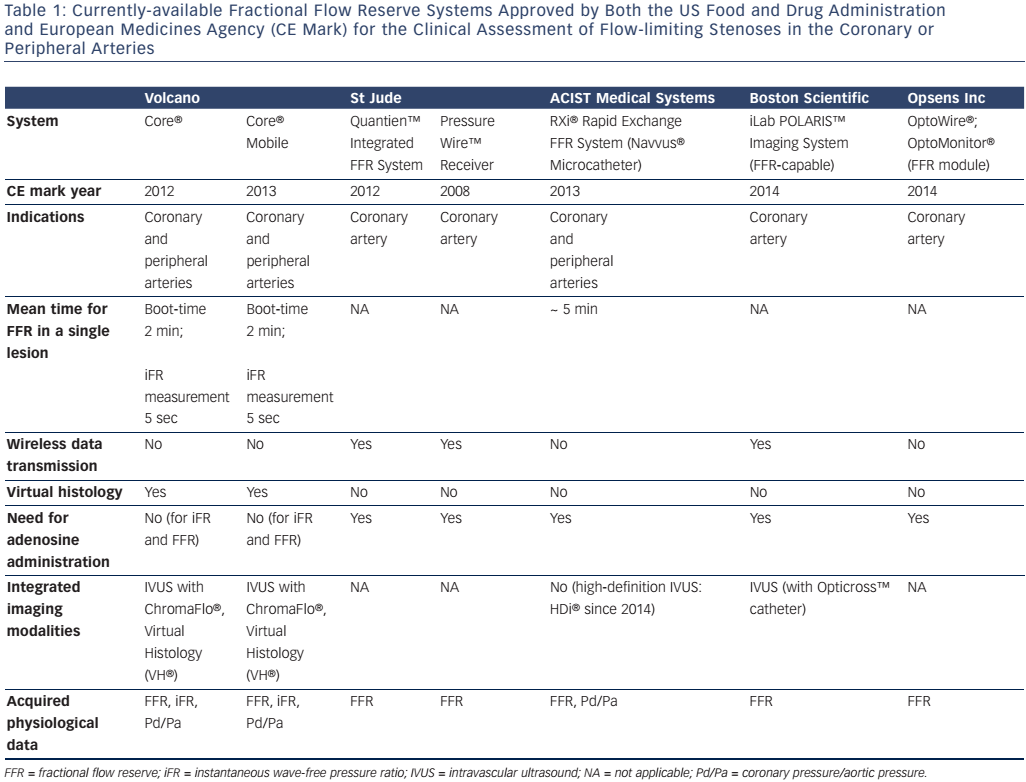
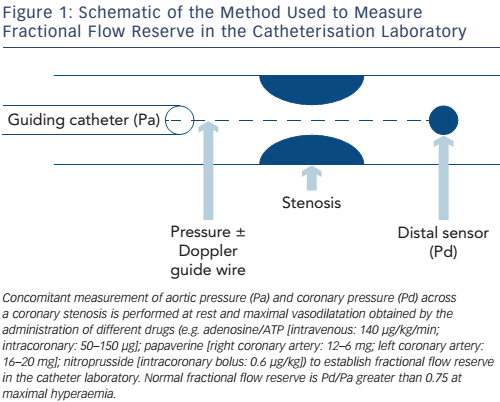
Aortic pressure is measured at the tip of a guiding catheter placed adjacent to the coronary ostium, and distal coronary pressure is recorded by a 0.014” guide-wire equipped with a pressure sensor 3 cm from its tip (see Figure 1). Some pressure-wires also have miniature intravascular ultrasound (IVUS) or Doppler probes and can be used to invasively measure CFR or provide anatomical information about the vessel wall by IVUS at the maximum vasodilation induced by adenosine.16 The most recent systems employ wireless technology and fibre-optic wires or catheters to improve pressure signal detection. A synopsis of the different FFR measurement systems available is provided in Table 1. Normal coronary physiology is associated with a FFR value of 1, while myocardial ischaemia occurs at a FFR of less than 0.75 with a diagnostic accuracy of 90 %.15 FFR is the gold standard method for detecting myocardial ischaemia in the cath-lab and should be performed routinely in intermediate coronary stenoses between 50 and 70 %. Its main advantages are:
- It has a well-defined cut-off point of 0.75 and a very narrow “grey zone” of between 0.75 and 0.80
- It is not influenced by systemic haemodynamic factors (such as blood pressure, heart rate and contractility)
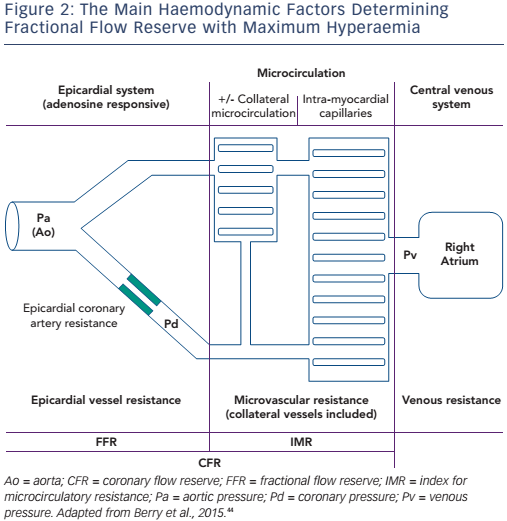
- In chronic stable ischaemic heart disease it does not depend on the status of microcirculation, as opposed to CFR; acute impairment by microemboli, spasm, compressing myocardial oedema or leukocyte margination in ACSs may be associated with false-negative (normal) FFR values
- It is influenced by the collateral flow, which may sometimes bring a significant blood supply to the distal myocardium, depending on a main narrowed epicardial vessel (see Figure 2)
- It is highly reproducible and easily obtainable after coronary angiography
- It has high spatial resolution in the case of multiple stenotic segments in the same vessel.
Current indications for FFR assessment when intermediate coronary stenoses are not present are related to the accurate identification of significant lesions in multivessel CAD, differentiation between the functional significance of focal and diffuse coronary atherosclerosis, establishment of the presence of a significant lesion in multiple consecutive stenoses in the same vessel, and finally assessment of the efficiency of collateral circulation. A FFR of less than 0.75 is superior in its detection of myocardial ischaemia to other tests used in practice (such as exercise testing, thallium perfusion scans and stress echocardiography), with a specificity of 100 %, sensitivity of 88 % and accuracy of 93 %.15
Recently an adenosine-independent technique was developed by a FFR system manufacturer and tested as a possible substitute for classic techniques that use maximal pharmacological hyperaemia. This technique is called the instantaneous wave-free pressure ratio (iFR). It has been determined that a wave-free period between the pressure wave in the aorta and distal microcirculatory pressure meets the criteria for FFR assessment. This occurs at 75 % in diastole 5 msec prior to the occurrence of the R wave. When compared to FFR, an iFR value of less than 0.89 correlated with an FFR of less than 0.80 in correctly identifying 83 % of significant coronary stenoses in the ADVANCE II study.17
There are some limitations of FFR measurements in lesions of intermediate severity, but they are relatively uncommon. Pressure damping by placing the pressure transducer on the wire too distally with no surrounding flow produces false-negative results (high or normal FFR). An inadequate dose of adenosine may also produce false-negative results.
In ACSs, FFR may also show normal values due to diffuse microcirculation impairment (or obstruction) in the distal myocardium. This generally improves over time, and when FFR is measured again the same vessel may prove to be significantly ischaemic with myocardial flow improvement.
Use of FFR to Determine Revascularisation in Stable CAD
Multiple clinical trials have demonstrated that revascularisation should only be performed on intermediate lesions in stable CAD if myocardial ischaemia can be documented by a FFR value of less than 0.75. It was also proven in the DEFERral of percutaneous coronary intervention (DEFER) trial that performing PCI on coronary lesions with a FFR greater than 0.75 leads to the same 5-year prognosis and chest pain prevalence as medical treatment.18 Patients with physiologically significant lesions with a FFR of less than 0.75 had the worst prognosis irrespective of revascularisation by PCI.18 Previous studies showed that the risk of a major adverse cardiac event at 1 year is 27 % when FFR is less than 0.75 compared to 9 % with a FFR greater than 0.75 in patients with multivessel CAD and intermediate stenosis.19
The Fractional Flow Reserve Versus Angiography for Multivessel Evaluation 2 (FAME 2) trial was stopped early by the Data and Safety Monitoring Board following the randomisation of only 888 patients (54 % of the planned enrolment group) after a mean follow-up of 7 months rather than 2 years because of a highly statistically significant reduction in the primary endpoint in the PCI group, due to a higher need for urgent revascularisation.20 In this trial, patients with stable CAD, intermediate coronary artery stenoses and at least one stenosis with a FFR of less than 0.8 were randomised to PCI with drug eluting stents plus optimal medical therapy versus optimal medical therapy alone. The primary endpoint was a composite of death, myocardial infarction and urgent revascularisation at 2 years. The composite endpoint was observed in 4.3 % of patients enrolled in the PCI group versus 12.7 % in the medical therapy group. The conclusion of the study was that FFR-guided PCI offers the best outcome when associated with best medical therapy in stable CAD patients. The decision to prematurely stop the trial was highly criticised, however, as there was no significant difference in the hard endpoints (death and myocardial infarction) between the groups. A second publication by the study investigators with complete 2-year follow-up showed a sustained 77 % reduction in the need for urgent revascularisation in the PCI group, but no difference in mortality or myocardial infarction.21 When all patients with periprocedural myocardial infarctions were excluded, statistical analysis showed a lower incidence of death or myocardial infarction in the PCI group (4.6 % versus 8 %; p=0.04).
Stable CAD patients with the worst prognosis are those with concomitant focal stenoses and diffuse disease sometimes combined in very long lesions with negative remodelling of the vessel.7 These are usually patients with long-standing diabetes or severe risk factors for CAD (i.e. familial hypercholesterolaemia). Physiological assessment in these vessels may be useful when deciding whether focal treatment is necessary, usually by PCI. Continuous pull-back of the pressure wire is performed by the operator while observing the FFR measurement at maximal hyperaemia; PCI and focal treatment is necessary if a sudden normalisation of pressure gradient is observed along the pull-back manoeuver along the diseased vessel.22 Continuous decrease in the pressure gradient along the investigated vessel is an indication for conservative treatment, although the prognosis remains reserved due to the severity of atherosclerotic disease.
One of the most useful indications for FFR measurement in stable CAD is multivessel disease. In the FAME trial, 1,005 patients with multivessel CAD were randomised to an angiography-based revascularisation strategy of all stenosis >50 % or a FFR of less than 0.8- based strategy for performing PCI.23 FFR-guided PCI was associated with a lower rate of total mortality, non-fatal myocardial infarction and repeat revascularisation (13.2 % versus 18.3 %, p=0.02). The incidence of angina was the same in both groups, however, with 80 % of patients being angina-free. Better resource use was observed in the FFR group, with a lower use of coronary stents. The mortality advantage was maintained at 2-year follow-up, with an 8.4 % prevalence of angina in the FFR group versus 12.9 % in the angiography-alone group.24
Another angiographical challenge that may be settled by the use of FFR is the use of revascularisation in patients with LM or right coronary artery ostial stenosis with downstream disease. When measuring FFR in these conditions, the operator needs to take extra care to avoid pressure damping by selectively engaging the vessel with the catheter tip, while using a continuous intravenous infusion of adenosine rather than an intracoronary bolus. FFR should be measured in both the LAD and circumflex (Cx) arteries by selective positioning of the pressure wire in the distal vessels. A FFR greater than 0.80 in 50 % LM artery stenosis was considered an indication for medical therapy alone, while in patients with the same angiographical stenosis of the LM artery and FFR of less than 0.80 CABG was performed.25 Only 23 % of the lesions with >50 % stenosis at angiography had a FFR of less than 0.80. Estimated survival rates at 5 years were the same in the non-physiologically-significant lesions of the LM artery (FFR greater than 0.80) as those with physiologically-significant lesions who were revascularised by CABG (89.8 % versus 85.4 %).
LM lesions with associated distal disease of the LAD and/or Cx arteries are more difficult to assess, as distal disease may influence the FFR of the LM artery stenosis. To appropriately assess the physiological significance of a LM stenosis, the pressure wire should be positioned in the non-diseased artery, either the LAD or the Cx. When both LAD and Cx arteries have atherosclerotic disease, a false-negative FFR measurement may be recorded. In these cases an observed FFR of between 0.81 and 0.85 corresponds to a real FFR of less than 0.80, and the artery requires revascularisation.26
Can Atherosclerotic Plaque Morphology Determine FFR in Stable CAD?
FFR differentiates between significant haemodynamic stenoses and non-ischaemia inducing lesions. It is less clear whether it can predict prognosis in patients with lipid-rich, large necrotic core plaques (thin cap fibroatheroma, TCFA) and allow treatment prior to the occurrence of an ACS. Some recent trials correlating FFR measurement with imaging studies of the vessel walls have demonstrated that only vulnerable, lipid-rich atherosclerotic plaques are associated with reduced FFR independent of the degree of luminal stenosis.27–29 In a study performed with both IVUS and FFR measurement in moderate coronary stenoses (between 50 and 70 %), only TCFA plaques were correlated with low FFR; larger, lipid-rich necrotic cores were correlated with lower FFR values.30 A normal FFR denotes a low likelihood of the presence of unstable, vulnerable plaques. It has been postulated that a normal FFR excludes not only haemodynamic impairment due to significant mechanical flow obstruction, but also unstable plaques with large necrotic cores, demonstrating that medical treatment alone is safe in all these cases.27
The COMBINEd Optical Coherence Tomography Morphologic and Fractional Flow Reserve (COMBINE (OCT-FFR)) is currently enrolling patients. It is a prospective trial comparing the outcomes in patients with diabetes mellitus and FFR-negative values in intermediate coronary artery stenoses with or without TCFA against FFR-positive patients treated with PCI.31 The trial’s primary endpoints are cardiac death, myocardial infarction, and clinically-driven target lesion revascularisation or hospitalisation for unstable angina at 18-months in non-TCFA patients versus TCFA patients both with normal FFR values. Secondary endpoints compare FFR-negative TCFA-positive patients with PCI-treated FFR-positive patients.
The Future of Coronary Physiological Measures in the Cath-lab
There is an intense debate about the use of physiological measurements in ACSs to guide non-culprit lesion revascularisation. Culprit lesion revascularisation without physiological assessment improves prognosis by reducing the risks of recurrent myocardial infarction and mortality.32 The long-term outcome for ACS patients is a return to the baseline risk of any CAD patient.
Coronary physiological assessment may eventually be used in ACS patients with no obvious culprit lesion at diagnostic angiography. This is not a common clinical situation as angiography identifies at least one culprit lesion in most ACS patients. When no obvious culprit lesion is identified, FFR measurements even in mild CAD may be useful, although the cause–effect relationship may be very difficult to prove and the decision to revascularise is debatable.
In ACS patients with clear culprit lesions, coronary physiology measurements of the culprit vessel may demonstrate serial improvement of blood flow due to progressive improvement of the resistance of the distal capillary bed (or index for microcirculatory resistance, see Figure 2).33 In these culprit vessels, blood flow measurements (using Doppler flow velocity or bolus thermodilution) after primary PCI are associated with clinical outcome.34,35 No clinical trials have yet demonstrated the prognostic value of flow measures after revascularisation for ACS; consequently, culprit vessel flow measurements provide some prognostic insights after an ACS, but their clinical use is still under debate.
Two small randomised studies have used FFR in non-culprit lesions to decide whether or not to revascularise ACS patients. The Fractal Flow Reserve Versus Angiography Guided Management to Optimise Outcomes in Unstable Coronary Syndromes (FAMOUS-NSTEMI) trial randomised 350 patients with at least one 30 % non-culprit stenosis to FFR (less than 0.8) or angiography to determine whether revascularisation by PCI or CABG was required. At 1-year follow-up, prognosis was the same for both groups, despite higher rates of revascularisation in the angiography-alone subgroup.36 In the Third DANish Study of Optimal Acute Treatment of Patients With STEMI: PRImary PCI in MULTIvessel Disease (DANAMI-3 – PRIMULTI) study, 627 patients with STEMI were randomised to FFR- or angiography-based revascularisation assessment in lesions with at least 50 % luminal stenosis in non-culprit vessels.37 All STEMI lesions were previously treated by PCI. Total mortality and non-fatal myocardial infarction levels were no different between the two groups at 1-year follow-up. There was a significant reduction in the revascularisation rate in the FFR-guided group at 1 year.
Whether or not to use FFR-based assessment of the need for revascularisation of non-culprit lesions in STEMI patients will be answered when results from the large-scale randomised COMPLETE trial are published in 2018.38 The trial will enrol 3,900 patients. Inclusion criteria will be related to a >70 % lesion or a 50–70 % stenosis with a FFR of less than 0.8 in a non-culprit lesion. The primary endpoint will be cardiovascular death and nonfatal myocardial infarction. Studies like COMPLETE with hard endpoints will be necessary in the near future to determine the value of FFR in deciding whether to revascularise non-culprit lesions in ACS.
Finally, the significance of medium-size jailed side branches when implanting stents in main coronary arteries can also be determined by FFR measurement. When an ostial side branch stenosis >50 % occurred after stenting the main vessel, the mean FFR in the side branch was 0.85±0.11 in vessels greater than 2 mm. One-third of lesions angiographically found to have stenosis more severe than 75 % were found to be physiologically significant by FFR.39
A New Non-invasive Option to Assess the Physiological Significance of CAD
New angio-CT techniques mean that FFR at maximum hyperaemia can now be non-invasively assessed using computational fluid dynamics.40 This method depends heavily on image acquisition and image-processing algorithms. It was even proposed that coronary angioCT coupled with FFR-CT be used to decide which patients with 30–70 % stenoses should go to the cath-lab, even when their Agatston calcium score was >400.41
The functional significance of coronary atherosclerosis could be obtained by a single non-invasive method; however, the results of the first three randomised trials comparing FFR-CT with FFR measured in the cath-lab using a pressure wire were not convincing.41–43 The non-invasive method is plagued by a low average diagnostic accuracy of about 80 % and it has been assessed in a rather low number of patients (see Table 2).
It has been proposed that the diagnostic accuracy of a non-invasive FFR-CT test should be compared with that of other non-invasive tests alone. There is hope that, with continuous improvement in technology and standardisation of the technique, FFR-CT will become a useful clinical tool.
Conclusion
CAD in stable patients is frequently associated with moderate coronary stenoses of between 50 and 70 %. The functional significance of these lesions should be assessed by FFR, and myocardial ischaemia should be demonstrated. FFR-guided PCI should always be used in stable CAD patients with moderate coronary stenoses as it is the best current therapy in these cases. Revascularisation is warranted in patients with stable CAD and a FFR of less than 0.75–0.80, either by PCI or CABG. Due to its functional significance, FFR is superior to other invasive anatomical investigation methods, such as IVUS or optical coherence tomography. When used concomitantly with FFR, it was demonstrated that low FFR values are obtained from moderate coronary artery stenoses that most frequently have large lipid-rich necrotic cores. The use of FFR is futile in stable ischaemic patients who have concordant high-risk stress tests and an angiogram showing a high-risk coronary anatomy (i.e. 50 % stenosis of the proximal LAD). There are great expectations that refinements to CT-angiography will enable the accurate determination of FFR and predict the need for revascularisation in stable CAD patients.