Dietary Patterns
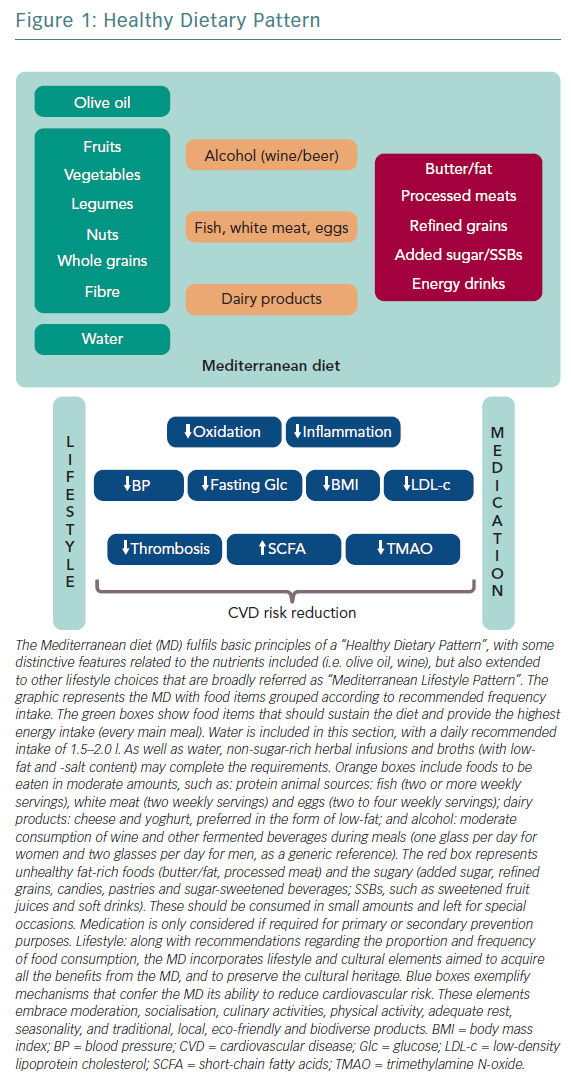
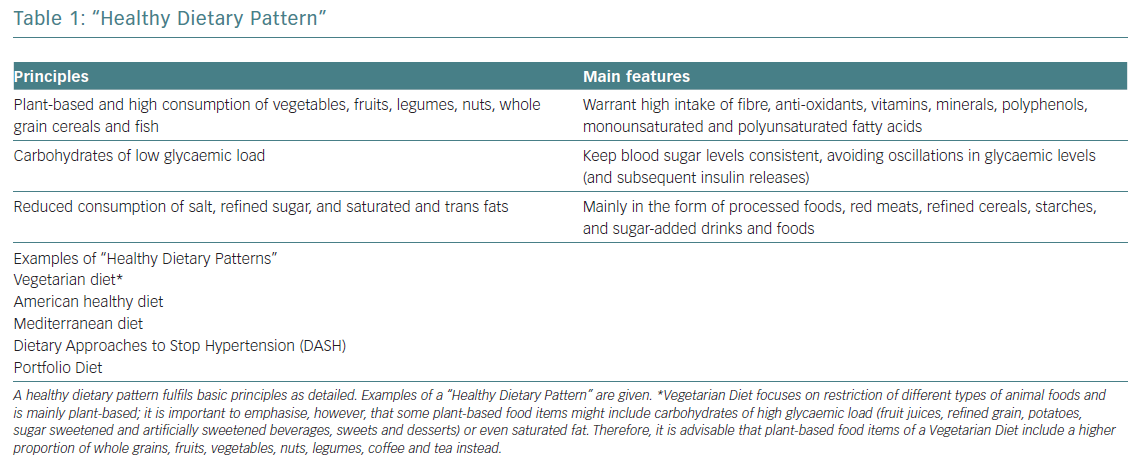
Several studies correlate healthy dietary patterns with lower plasma concentrations of pro-inflammatory markers.1 These healthy dietary patterns support greater benefits than the potential effects of a single nutrient supplementation. The current body of evidence shows that healthy dietary patterns share similarities, shown in Figure 1.2 These features fit with the report of the most recent workshop convened by the World Heart Federation, and three models are recommended by the Unites States Dietary Guidelines Advisory Committee: Mediterranean diet (MD), American healthy diet and vegetarian diet (VD).3,4 The latter is a type of plant‐based diet that restricts different types of animal foods (meat, poultry or fish), and has been associated with a lower risk of cardiovascular (CV) risk factors (obesity, hypertension, type 2 diabetes) and coronary heart disease (CHD).5,6 However, VD, as a concept, focuses more on the exclusion of animal sources of food than the quality of plant foods; this raises some heterogeneity and deserves further research to assure the features of a heart healthy diet.7 Thus, recent studies tried to cluster different subtypes of VD according to the frequency of intake of three food groups: healthy plant foods (whole grains, fruits, vegetables, nuts, legumes, coffee, tea), less healthy plant foods (fruit juices, refined grain, potatoes, sugar sweetened and artificially sweetened beverages, sweets and desserts) and animal foods (animal fat, dairy, eggs, fish or seafood, meat and miscellaneous animal foods) given their associations with chronic conditions.8 Diets with higher intake of healthy plant foods and lower in animal foods were associated with a lower risk of incident CV disease (CVD), CVD mortality and all‐cause mortality.5–7 No true association was found with less healthy plant‐based diets and CVD or all‐cause mortality.7 Therefore, health implications of diets high in refined carbohydrates and sugar, and low in fruits, vegetables and animal foods must be acknowledged when assessing individuals with a VD.
Other therapeutic diets, such as the Dietary Approaches to Stop Hypertension (DASH) and the Portfolio diets recommended in the Canadian Cardiovascular Society guidelines, also emphasise the principles of a healthy diet.9,10
The MD and the DASH diet are probably the best-studied dietary patterns in relation to CVD prevention. Both may improve downregulation of low-grade inflammation and better control of bodyweight, further controlling other risk factors, and ultimately are correlated with lower numbers of clinical events.5,11
Mediterranean Diet
The MD is defined as the traditional dietary pattern found in the early 1960s in Greece, Southern Italy, Spain and other olive-growing countries of the Mediterranean basin.12 It is a frugal diet that fulfils the definition of “healthy diet” (Table 1), with some distinctive attributes (Figure 1): olive oil the principal source of fat, moderate intake of wine (mainly wine during meals), fish, seafood, poultry, eggs and dairy products (cheese and yoghurt, preferred in the form of low fat), and low consumption of red meat.
All kinds of olive oil (virgin, extra virgin olive oil and refined olive oil) contain oleic acid as the main fat, but only unrefined olive oil (virgin and extra virgin olive oil) contain tocopherols, phytosterols, monounsaturated fatty acids (MUFA) and several bioactive polyphenols (hydroxytyrosol, tyrosol, oleocanthal, resveratrol), with postulated anti-atherogenic and anti-inflammatory properties.13–15
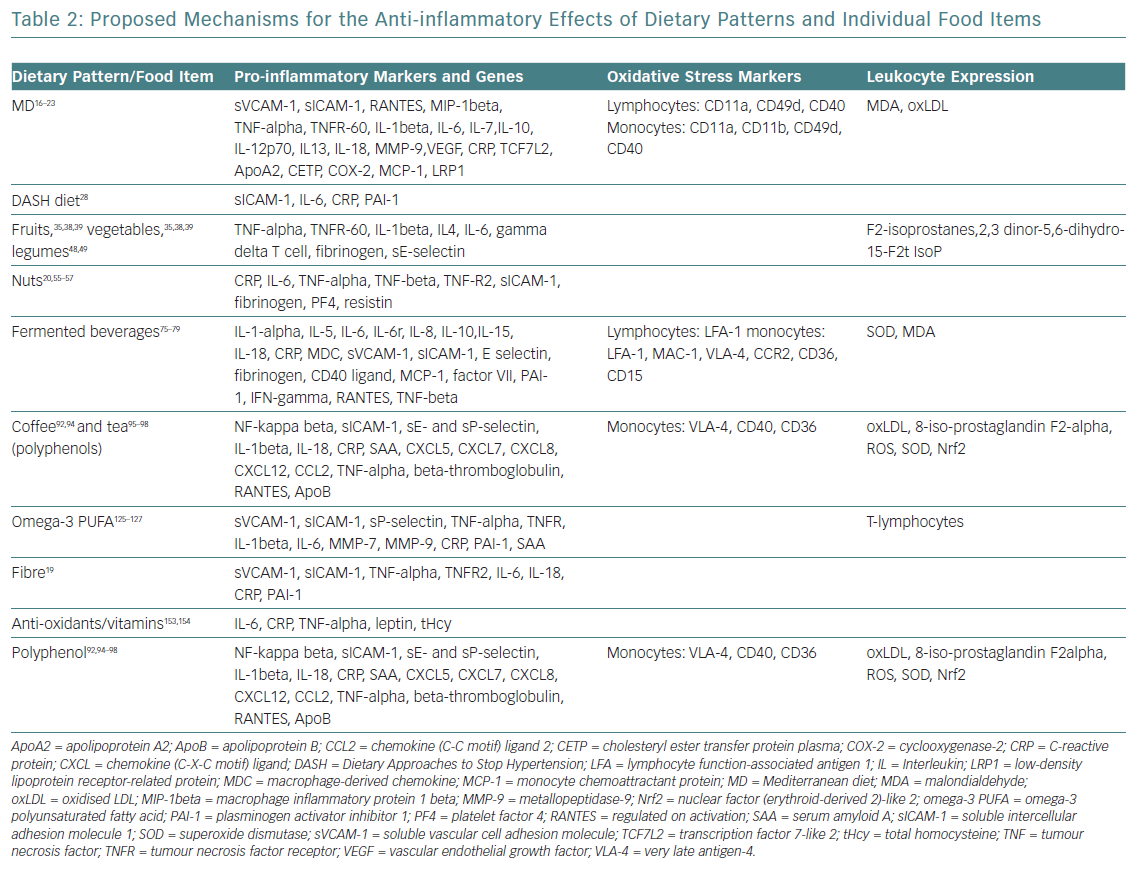
The evidence supporting CVD benefits is large, strong and consistent, in terms of clinically meaningful rate reductions of CHD, ischaemic stroke and total CVD. Until now, these benefits had been attributed to improvements in blood pressure, lipid profile, glucose metabolism, arrhythmic risk or gut microbiome.5 However, vascular anti-inflammatory effects have recently been hypothesised as a possible mechanism that links MD and low CVD prevalence (Table 2).16–19
This hypothesis was confirmed by the Prevención con Dieta Mediterránea (PREDIMED) findings on MD mechanisms: modulation of the expression of adhesion molecules in leukocytes; improvements in the circulating levels of soluble adhesion molecules, cytokines, chemokines and macrophage inflammatory proteins; and plaque stabilisation after 3 months, and 1, 3 and 5 years of intervention.20 Epigenetic studies of the MD reinforce these results, with proven influence on the methylation status of peripheral white blood cell genes, interactions among MD and the expression of other molecules (cyclooxygenase-2, interleukin-6, apolipoprotein, cholesteryl ester transfer protein plasma), transcription factors and gene polymorphisms (Table 2).21–23
The authors of the PREDIMED study in 2013 recently retracted the original publication as a result of an error in the randomisation procedures affecting a portion of participants included.24 The authors re-ran the analyses omitting 1,588 participants from 7,447, and published a corrected version that showed no significant changes in the results of the trial.24,25 In both the original and republished study, the incidence of CVD in the MD groups was lowered by approximately 30% when compared with the control diet.20,24,25 Therefore, the overall conclusion remains unchanged, and PREDIMED remains the largest dietary intervention trial to assess the effects of the MD on CVD prevention.
Dietary Approaches to Stop Hypertension Diet
The DASH model follows the “healthy diet” pattern (Figure 1), with critical emphasis on a low intake of sodium and refined grains.26,27 Focusing on inflammatory markers and oxidative stress, several studies have shown the protective effect of the DASH diet on CVD (Table 1) mediated by significant reductions of high-sensitivity C-reactive protein concentrations. Cross-sectional analysis evaluating potential associations between dietary quality (DASH dietary quality score), adiposity, and biomarkers of glucose metabolism, lipid profile and inflammation reveal that a higher adherence to the DASH dietary pattern significantly improves adiposity measures, and lowers concentrations of pro-inflammatory, pro-thrombotic and pro-atherogenic markers.28 Improvements in lipoprotein profile and glucose homeostasis are also achieved.
It has also been shown that a DASH diet increases plasma renin activity and serum aldosterone levels in response to blood pressure reductions.29 The effect of sodium intake on blood pressure differs by genotype at the angiotensinogen, beta2-adrenergic receptor and kallikrein loci.29,30 These findings have implications for understanding the mechanisms through which diet affects blood pressure, the heterogeneity of these effects, and the extent to which dietary and pharmacological interventions can modulate genetic predisposition.29,30
Individual Food Items
There are some specific dietary guidelines that are exclusively food based.31–33 Comprehensive dietary modelling is undertaken to ensure nutrient reference values, including targets for sodium, and saturated and trans fat. This section develops some of the features of each of the individual food items and its possible relationship to CVD risk reduction.
Fruits and Vegetables
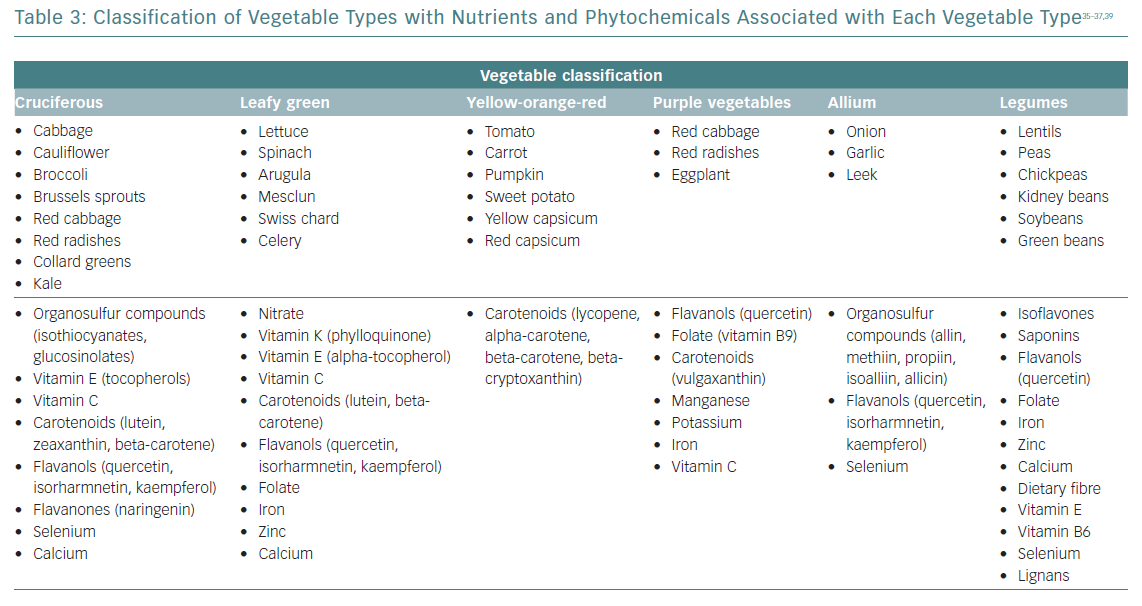
Daily consumption of multiple servings of both fruits and vegetables is strongly and widely recommended, since its total intake has been inversely associated with CVD risk, and seems to be the healthiest and most beneficial source of anti-oxidants for CVD risk reduction.34 The benefits for subgroups have been less studied, and may vary considerably according to their phytochemical and micronutrient composition (Table 3).35–37 Some of the anti-inflammatory mechanisms proposed for fruits and vegetables are summarised in Table 2. Anthocyanins (a subclass of flavonoids) found in blueberries, strawberries, raspberries, red cabbage, red radishes and eggplant have potent anti-inflammatory properties: free radical scavenging, endothelial nitric oxide (NO) regulation, endothelial function modulation and influence on glucose metabolism.35–39
Cruciferous vegetables have been associated with vascular benefits due to their potential to release nitrite via the enterosalivary nitrate–nitrite–NO pathway. Dietary nitrates are secreted by the salivary glands and reduced to nitrite via the action of commensal oral bacteria. Salivary nitrite levels can increase >1,000‐fold greater than in the circulation.40 Among leafy green vegetables, nitrate concentrations are most appreciable in spinach, arugula, mesclun, lettuce and Swiss chard.41,42 The European Food Safety Authority has set the Acceptable Daily Intake for nitrate at 3.7 mg/kg (approximately 260 mg for a 70-kg adult).41 A recently published systematic review and meta-analysis reported that intakes of dietary nitrate were significantly associated with a reduction in resting blood pressure, improved endothelial function, reduced arterial stiffness and reduced platelet aggregation.43
The amount of vegetable intake recommended in dietary guidelines varies globally, but is usually around five or six servings/day (375–450 g/day). For vegetable types, leafy green vegetables, cruciferous vegetables and tomatoes were inversely associated with CVD risk in non-linear dose–response analyses. The greatest CVD benefits were observed at intakes of ≥200 g/day for cruciferous vegetable, ≥120 g/day for leafy green vegetables and ≥200 g/day for tomatoes.44
Daily recommendations on fruit intake range from 100 to 300 g (200 g/day). However, it must be taken in to account that the process of juicing concentrates calories, risking excess energy intake and loss of fibre. There are few studies evaluating the clinical benefits of vegetable juicing versus raw or cooked forms. Until comparative data become available, whole food consumption is preferred, with juicing primarily reserved for situations when daily intake of vegetables and fruits is inadequate. Guidance should be provided to maintain optimal overall caloric intake and to avoid the addition of sugars (e.g. honey) to minimise caloric overconsumption. In addition, there is no evidence of CV benefit with the addition of high-dose antioxidant dietary supplements if these intakes are warranted.
Legumes
Legumes are seeds with complex matrices rich in nutrients and chemicals, carrying a high caloric density that makes them an affordable and sustainable source of protein and fibre. Various effects on CV risk factors provide evidence for CV prevention. These foods have a low glycaemic index, and reduce glycaemia and postprandial insulinaemia, favouring diabetes prevention, especially in the context of a MD.45 Legumes have a hypocholesterolaemic effect (lowering both LDL and triglycerides), but their presumed effect in reducing blood pressure has not been consistently proven.46,47
Legumes are a good source of protein, starch, isoflavones, vitamin B6, folate and iron. The anti-inflammatory effects of this food group are frequently included with vegetables, which makes it difficult to separate its own mechanisms on pro-inflammatory/oxidative stress markers and leukocyte expression (Table 2). However, significant reductions of high-sensitivity C-reactive protein, interleukin-6 and tumour necrosis factor-alpha with a legume-based diet have been demonstrated, independent of caloric intake or weight change.48,49
Based on these beneficial properties, legumes should be part of any cardiometabolic healthy diet, with a daily intake of around 50–100 g (140–180 kcal/day).50
Nuts and Seeds
Nuts and seeds (almonds, hazelnuts, walnuts, pistachios, cashews, macadamias, pinions, peanuts etc.) are peculiar vegetables with a high fat content (usually exceeding 50% of energy) mainly containing unsaturated fatty acids (UFA), such as oleic MUFA (almonds, hazelnuts) or n-6 polyunsaturated linoleic and n-3 as alpha-linolenic acid (in nuts). Although peanuts are actually vegetables, their composition and UFA content assimilates them to nuts. Nuts and seeds contain other bioactive compounds: L-arginine, soluble fibre, vitamin E, phytosterols, polyphenols, anti-oxidants, potassium, calcium and magnesium. Numerous large prospective cohort studies have demonstrated reductions in CVD morbidity and mortality with the consumption of nuts and seeds.51,52 Mechanisms proposed for favourable CVD outcomes are likely mediated by dose-dependent hypocholesterolaemic effects and improvements of glycaemic profile.53,54 The PREDIMED study provided first-class evidence that regular nut consumption halves the incidence of diabetes, and reduces the incidence of CVD by 30%.20 Additional benefits are derived from the role on oxidative stress, inflammation and vascular reactivity through modulation of inflammatory and oxidants mediators (Table 2).20,55–57
Although the benefits are accepted, there is no standard recommendation for nuts inclusion on dietary patterns. Following a MD, daily supplementation with a serving of mixed nuts (i.e. 15 g walnuts, 7.5 g almonds and 7.5 g hazelnuts) would be enough to get the desired CV effects. Caution must be taken at >75 g, because of the risk of excess caloric intake.
Grains and Tubers
Grains are the largest source of energy in almost all diets worldwide. The bran and germ layers present in whole grains are rich in fibre, lignans, micronutrients, fatty acids and other phytonutrients.58 Depletion of these nutrients during the milling process partially explains why whole grain consumption is generally related to higher satiety and a lower glycaemic response compared with refined grains.59 High intake of whole grains has been associated with reduced risk of CHD disease, type 2 diabetes and overall mortality.60 Refining grains, in contrast, causes major loss of nutrients and fibre, which has important health implications, including adverse metabolic effects, weight gain, increased risk of CVD and overall mortality.60–62
There are few data linking gluten and CHD. People with coeliac disease or gluten sensitivity might have inflammatory mechanisms more related to zonulin release into the gut than to gliadin, and these altered pathways could predispose to type 2 diabetes and even CHD.63,64 However, there is no current evidence supporting a link between gluten consumption and CHD, and should not be restricted in people without coeliac disease or gluten sensitivity.65
Roots and tubers (the so-called starchy vegetables) are a good source of starch, which may help maintain a healthy gut.66 Gut dysbiosis is associated with intestinal inflammation and has been linked to the development of CVD.67 However, there is no evidence that restoring gut dysbiosis with tubers improves CV outcomes. Intake of potatoes, for instance, provides a large amount of rapidly absorbed carbohydrate (glycaemic load), and its daily consumption has been associated with increased risk of type 2 diabetes, hypertension, weight gain and even CHD (especially consumed in the form of “French fries”).68–71
Evidence does not support strong recommendations on the specific proportion of energy intake from carbohydrates, but keeping this to <60% of energy appears desirable, and consumption of whole grains is emphasised. This would be about 232 g/day of whole grains, and 50 g/day of tubers and starchy vegetables (with a limit of 100 g/day of tubers and starchy vegetables).
Fish and Seafood
Fish intake has been associated with reduced risk of CVD, mainly attributed to the particular properties of omega-3 fatty acids (O3FA), which are abundant in fish composition.72,73 O3FA are precursors of eicosanoids, a large component of the central nervous system, a structural element of every cell of the body and a regulator of cardiac rhythm. They are thought to reduce arrhythmias, thrombosis, inflammation and blood pressure, and favourably modify the lipid profile.74 An average weekly intake of 2 g of O3FA in fish might reduce CVD risk by more than one-third.72 Although O3FA from plant sources (specifically alpha-linolenic acid) have been associated with reduced risk of CVD disease and had been proposed as an alternative source to substitute fish, the quantity required is not clear.
Beverages
Alcohol
Alcoholic beverages contain ethanol (ethyl alcohol) and are classified by the elaboration process as: fermented (by alcohol fermentation; <15% alcohol content): red or white wine, beer or cider; and distillate (by alcohol distillation; 20–60% alcohol content): spirits, such as cognac, whiskey, gin, vodka and rum, and liqueurs flavoured with fruits, herbs or spices.
Fermented beverages are believed to provide a greater CV protection than distillate beverages, especially red wine. Its higher polyphenol content favourably modifies oxidation and inflammation parameters related to arteriosclerosis by different pathways: higher NO availability (improving endothelial function), increases in HDL cholesterol levels and anti-aggregation/profibrinolytic/anti-inflammation properties (Table 2).75–79 Beer is another type of fermented beverage with moderate polyphenol content that has cardioprotective effects comparable to wine.80,81 Both alcoholic and non-alcoholic beer improve inflammatory biomarkers profile, homocysteine and folic acid levels (Table 2).
Alcohol intake and CVD risk show a “U-shaped” relationship, with both abstainers and heavy drinkers carrying a higher risk than moderate drinkers.82,83 Adverse effects of (often heavy) alcohol consumption include a higher risk of atrial fibrillation, non-ischaemic dilated cardiomyopathy and long-term weight gain.84–86 In addition, alcohol is causally linked to upper aerodigestive tract cancers (oral cavity, pharynx, larynx, oesophagus), and those of the colon, liver and female breast. Associations exist for many other types of cancer, but the precise role of alcohol requires further research for it to be fully disentangled from ecological and lifestyle factors.87
By definition, a standard drink contains 14 g of ethanol (17 g of pure alcohol). This equates to 350 ml of beer (5% ethanol), 150 ml of table wine (12% ethanol), or 45 ml of hard liquor or distilled spirits (40% ethanol).88 Although the exact nadir of risk depends on sex, age, ethnicity and baseline disease, it seems that consuming one or two daily drinks derives the lowest risk (2 for men; 1–1.5 for women).89,90 This would be the daily intake recommendation, mainly in the form of fermented beverages.
Coffee
Coffee is one of the most widely consumed beverages in the world, representing the liquid extract of coffee beans. It contains many active compounds responsible for its bitter taste, and conferring anti0oxidant and anti-inflammatory actions.
Several mechanisms contribute to the sustained CV health effects of coffee. However, the response of each individual component varies, and might interfere with others in a complex relationship. Various genetic polymorphisms affecting caffeine metabolism (i.e. cytochrome P450 1A2 variants), receptor-mediated effects (i.e. adenosine receptors) or non-receptor mediated effects (e.g. low catechol-O-methyltransferase activity) may influence an individual’s response to caffeine. An increased risk of CHD or MI has been reported only among individuals with a genotype associated with slow caffeine metabolism (CYP1A2*1F instead of CYP1A2*1A), or low catechol-O-methyltransferase activity genotype (low catecholamine metabolism).91 The infusion of coffee maintains a high concentration of potassium, magnesium, vitamin E, niacin, polyphenols (mainly chlorogenic acid), micronutrients, lignans and phytochemicals. Chlorogenic and caffeic acids improve the anti-oxidative status of the body by slowing down the process of inflammation, which protects from the hazardous effect of free radicals and against endothelial damage. There is no scientific association with blood pressure elevation and, in turn, it actually lowers diabetes risk in a dose-dependent manner.92
Unlike filtered coffee, some components present in unfiltered coffee (cafestol and kahweol) raise serum lipids.93 Whether these components are involved in the deposition of LDL cholesterol is still debated. Usually, consumption of three or four daily cups of coffee leads to a small increase in HDL cholesterol. The effect on LDL is more complex, since the resistance of LDL to oxidative modification increments significantly after drinking coffee, but the LDL concentration does not (or at least, not significantly).94 Therefore, regular consumption of coffee (3–5 cups/day, which corresponds to a coffee polyphenol intake of 101–337 mg/day) can be recommended based on its ability to lower CVD risk.93
Tea
Tea contains a significant amount of flavonoids and polyphenols, considered the most abundant dietary anti-oxidants present, and responsible for a wide range of health effects in the prevention of CVD.95 Polyphenols delay progression of atherosclerosis through several mechanisms: regulation of signalling–transcription pathways (including downregulation of pro-inflammatory cytokines) and anti-oxidant systems (enhanced NO production), prevention of leukocyte migration/plaque infiltration, and reduction of adhesion molecules, among others (Table 2).96 Both short- and long-term tea consumption have shown to improve endothelium-dependent flow-mediated dilation, reverse endothelial vasomotor dysfunction in CHD patients and are associated with favourable changes on lipid profile.97–99 These effects translate into a lower risk of developing CHD and major cardiac events, even all-cause mortality.100,101
The evidence for a favourable CVD profile is based on regular tea consumption (3–5 cups/day) without added sugars, sweeteners or milks and creams (both animal and plant-based), and that should be the proper way to tackle any recommendation on this beverage.
Dairy Products
A growing body of nutritional science highlights the complex mechanisms and pleiotropic pathways of cardiometabolic effects of dairy products (i.e. milk, yogurt and cheese) that may be mediated by specific proteins (whey and casein proteins), amino acids (leucine, isoleucine and valine), medium-chain and odd-chain saturated fats, UFA, branched-chain fats, natural trans fats, probiotics, vitamin K1/K2, and calcium, or by processing methods (fermentation/homogenisation). These intricate processes translate into divergent conclusions regarding CVD: although systematic reviews and meta-analyses show either neutral or a favourable association between dairy intake and CVD-related outcomes, other studies associate dairy fat with a unfavourable risk profile that can be reversed by replacing fat from dairy products with polyunsaturated fatty acid (PUFA) or vegetable fat.102–108 In cohorts utilising objective biomarkers, higher blood levels of dairy fatty acids are consistently associated with a lower incidence of diabetes and neutral/favourable CVD risk profile. Reduced fat dairy products remain a convenient source of some essential vitamins and minerals, and high-quality protein. Obtaining these compounds should not be routinely based on supplements, since fermentation processes and probiotics are a relevant component in the biological pathways and clinical effects of these foods.109 In fact, the VITamin D and OmegA-3 TriaL (VITAL) trial has recently shown that diet supplementation with non-dietary vitamin D did not result in a lower incidence of invasive cancer or CV events than a placebo, which highlights the relevance of metabolic pathways in the effects observed with dairy products.110
Although further investigation is required, based on available evidence, the empiric recommendation on reduced-fat in place of regular- and high-fat dairy is adequate, and is included in MD and DASH dietary patterns (average 250 mg/daily, yielding 150 kcal of caloric intake).
Eggs
The high cholesterol concentrations found in eggs (200–230 mg/egg; 350–385 mg/100 g) led to the widespread recommendation of limiting egg intake in fear of subsequent increases in total and LDL cholesterol.111 However, eggs are rich in amino acids and several micronutrients that might interplay for the net effect on cholesterol levels and its clinical impact. Clinical studies reveal that cholesterol increases are discrete, with interindividual variability, and are coupled with slight elevations in HDL cholesterol that favour the development of large and low-atherogenic LDL particles.112 In fact, even daily egg consumption is not clearly associated with incident CVD in general populations and might reduce stroke risk.113–115
However, US dietary guidelines raised controversy because of apparently contradictory statements, saying that ‘cholesterol is not a nutrient of concern for overconsumption’, but that ‘individuals should eat as little dietary cholesterol as possible’.7
A recent study involving pooled individual data from six prospective US cohorts found that egg consumption was associated with an increased incidence of CVD and death.116 However, the association was possibly biased and inaccurately proven. A recent meta-analysis and systematic review found no association between egg intake and CHD or total mortality, but, in contrast, lower risk of mortality from stroke.117 Egg consumption has been also associated with hypertension, type 2 diabetes and markers of glucose homeostasis.117,118
The debate on the role of eggs for CVD prevention would remain largely moot until further data (including the genetic basis of cholesterol intake on CVD risk) are clearly defined. In the meantime, the importance of following evidence-based dietary recommendations, such as limiting intake of cholesterol-rich foods, should not be dismissed. Cardiometabolic effects can be derived from the consumption of up to two or three eggs per week, even in people with diabetes.
Oils, Butter and Fats
Primary types of dietary fat include saturated fat (SFA), UFA (including MUFA and PUFA) and trans fatty acids. Only dietary trans fatty acids intake demonstrates a consistent and strong association with adverse CVD outcomes. SFA have received widespread controversy, as the increases of total and LDL cholesterol levels undoubtedly translate into either neutral or harmful CVD outcomes in different clinical trials and metanalyses.119–122 It must be acknowledged that SFA are diverse compounds with variable effects on CHD risk depending on many factors apart from the dietary SFA intake, such as the SFA status, biomarkers and the carbon length of the SFA.123 Genetic factors contribute to the risk of CHD related to the dietary intake of C-18 fatty acid. It is advisable to replace long-chain fatty acids (LCFA) with PUFA, MUFA, short-chain fatty acids, whole grains and plant proteins.123,124
Mechanisms for the benefit with long-chain O3FA derived from fish oil might include improvements in the lipid and lipoprotein profile, oxidation, thrombosis, platelet aggregation, endothelial function, blood viscosity, membrane fluidity and plaque stability, modulation of concentration/expression of pro-inflammatory markers (adhesion molecules, cytokines etc.), and immune cells (Table 2).125–127
Noteworthy, benefits of the supplementation with these fatty acids remain to be confirmed.128,129 Although icosapent ethyl administration in patients with elevated triglyceride demonstrated significant reduction in CVD outcomes, the addition of O3FA did not result in a lower incidence of major CV events or cancer than placebo in a primary prevention trial.110,130 In addition, it is not possible to recommend the safest amount of dietary consumption of SFA, especially LCFA, at this time, but studies suggest it should be well below 9% of total caloric intake.123
Coconut oil is 92% SFA, predominantly lauric acid C12:0 and myristic acid (C14:0). Since only 4% of coconut oil has short-chain fatty acids (C-10 or less fatty acids), it acts mostly as a LCFA, with direct portal vein absorption and is highly soluble in water.131 However, there is a lack of prospective studies on CV, and the current literature raises mixed effects on serum lipids and the content of LCFA. In addition, replacement of coconut oil with PUFA and MUFA seems to reduce CHD risk.64 Therefore, this yet scarce evidence does not currently support coconut oil use for the prevention or treatment of CVD, and general recommendations on SFA intake (limited to <9% of total energy intake) should prevail.7,123
Meat
The intake of meat has increased in industrialised countries, and actually constitutes the basic component of meals. Although general meat consumption has been reported to be associated with all-cause and specific-cause mortality, the type of meat considered (red, white, processed) might redefine these associations. Red meat and processed meat may increase the risk of all-cause and CV mortality by means of several components that boost CV alterations.132,133 Various red meat-associated agents have been invoked, including SFA, high salt intake, trimethylamine N-oxide generation by microbiota and environmental pollutants contaminating red meat, none of which are specific to red meat. For instance, it has been demonstrated that residues of organochlorine pesticides are present in red meat at concentrations close to the WHO maximum recommendations. Epidemiological evidence and systematic reviews support an association of pesticide exposure with CVD and CV mortality (MI, cerebrovascular disease). These associations might be mediated via oxidative stress and inflammation pathways.134
Other human-specific hypotheses associated with red meat are also plausible, such as infectious agents (viruses) or xenoautoantigens (triggered by metabolic incorporation of a non-human sialic acid N-glycolylneuraminic acid into the tissues of red meat consumers).133 N-glycolylneuraminic acid incorporation from red meat can induce xenosialitis in vascular endothelium, and may contribute to red meat-induced aggravation of atherosclerosis and CVD. Despite all this circumstantial evidence, further research is required to confirm that this process is actually pro-atherogenic in vivo and thus a major causative factor in the development of CVD in humans.134
Very little has been reported about the impact of white meat intake on health; the interpretation of such effects is an arduous task, as individuals consuming more white meat are, at the same time, consuming less red meat. Findings obtained from meta-analyses are weak, and do not report increments on all-cause or CV mortality with 100 g daily consumption.135,136 Therefore, this could be a reasonable recommendation until more studies assessing the effect of white meat consumption on mortality are conducted, and there is no current evidence to support the choice of white over red meat in terms of CV risk reduction.
Added Sugar and Sugar-sweetened Beverages
The first associations between excess intake of added sugars, metabolic abnormalities and CV risk surfaced in the 1950s, but were eclipsed until recently (2014) by the belief that an excess intake of SFA was the key dietary factor.
Sweeteners are mainly being consumed as sugar substitutes that can be classified as nutritive sweeteners (polyols or sugar alcohols) and non-nutritive substitutes. Although almost 75% of packaged foods contain added sugars, sugar-sweetened beverages (SSBs; soda, sweet teas, fruit drinks) account for half of all added sugar intake.137–139 The effects of non-nutritive substitutes, both artificial sweeteners (acesulfame K, aspartame, cyclamate, saccharin, neotame, advantame and sucralose) and natural sweeteners (NSSs; thaumatin, steviol glucosides, monellin, neohesperidin dihydrochalcone and glycyrrhizin) are conflicting.140 NSSs interfere with glucose and energy homeostasis; alter leptin levels; adversely modify lipid profile, inflammatory factors, circulation and composition of gut microbiota; and decrease satiety, consequently increasing the risk of CHD, MI, cerebrovascular accident and vascular death.64,137,140–146
While some studies report an association between NSS use and reduced risk of overweight, obesity and type 2 diabetes, other studies suggest that NSS use could increase all of them, and the risk of metabolic syndrome, CHD and cancer.64 This association might be influenced by gene–SSB interactions.137 In particular, intake of SSBs can exacerbate the effects of chromosome 9p21 variants (i.e. rs4977574), considered the most robust genetic markers on CHD.147 The clinical and epidemiological data available at present are insufficient to make definitive conclusions regarding the benefits of non-nutritive substitutes in displacing caloric sweeteners as related to energy balance, maintenance or decrease in bodyweight and other cardiometabolic risk factors.
Based on the previously mentioned evidence, numerous expert bodies have now made recommendations to limit dietary added sugar intake to <10% of calories, and preferably <100 calories daily for women and <150 calories daily for men. Clinicians should recommend careful selection of foods with no or low amounts of added sugars in any form, and elimination of SSB. Patients should also be taught how to read nutrition labelling for added sugars.148,149
Other Nutrients and Bioactive Compounds
It is important to focus on the potential benefits of the intake of specific nutrients to avoid possible deficiencies of these nutrients, which can lead to the development of atherosclerotic disease.
Fibre
The health benefits of dietary fibre intake are indisputable, while a deficiency of fibre intake is associated with CVD development.150 The implicated mechanisms include decreased glucose/cholesterol absorption, downregulation of expression of oxidative stress-related cytokines or the inflammatory response mediated by gut microbiota exposed to fibre.19 The influence of long-term fibre intake on gut microbiota responsiveness to specific interventions is now becoming apparent. Increased fibre intake has been shown to improve certain metabolic parameters associated with obesity and its comorbidities (glucose homeostasis, serum cholesterol levels, blood pressure), particularly in conjunction with energy-controlled dietary regimes.151 The association between dietary fibre intake and risk of CHD has been studied through meta-analysis showing a significant dose–response relationship, especially for fibre from cereals and fruits.152
Bioactive Compounds
Recent research has identified the bioactive compounds that contribute to the beneficial effects of foods rich in anti-oxidants (beta-carotene, vitamin C, vitamin E, selenium) and their potential mechanism: reducing endothelial cells damage, improving the production of NO and inhibiting oxidation of LDL cholesterol (Table 2).153,154
Although the supplement industry began to promote the benefits of anti-oxidant supplements long before scientific evidence was available, recent multiple subsequent trials have reported either neutral or negative results for vitamin E, beta-carotene, O3FA, vitamin D or multivitamin supplementation.110,155–160 Thus, although foods rich in anti-oxidants at physiological levels appear to be have health benefits, further investigation will be necessary to better define the role of anti-oxidant supplements in health promotion.161,162
Polyphenols
Polyphenols are the most abundant dietary anti-oxidants present in most plant origin foods and beverages, which possess a wide range of health effects in the prevention of CVD.95 The most relevant food sources have been previously described (fruit and vegetables, red wine, black tea, coffee, extra virgin olive oil, nuts), and their potential anti-inflammatory role has been summarised.163
Conclusion
We defined a healthy dietary pattern taking into consideration nutritional adequacy as recommended by most dietary guidelines. A focus exclusively on food groups does not incorporate added fats, sugar and other constituents, and the interplay of intermediate risk factors within inflammation processes. The healthy dietary pattern we proposed allows for flexible, global application of these criteria (Table 1), with foods and amounts tailored to the preferences and cultures of different populations.