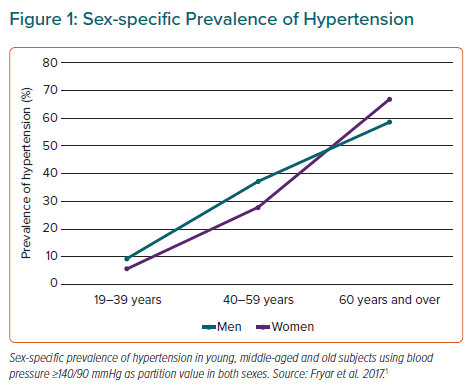
Sex Differences in Hypertension Over the Course of Life
The WHO has estimated that one in four men and one in five women worldwide have hypertension, identified as a systolic blood pressure (BP) ≥140 mmHg and/or a diastolic BP ≥90 mmHg in both sexes. Using this definition, the NHANES performed in the US in 2015–16, found a higher prevalence of hypertension in men than women among adults aged 19–39 years (9.2% versus 5.6%) and 40–59 years (37.2% versus 29.4%), whereas hypertension was more common in women among adults aged 60 years and over (66.8% versus 58.5%; Figure 1).1
The observed age-related sex difference in hypertension prevalence has traditionally been related to hormonal changes during menopause in women, and associated loss of beneficial vascular health effects, reduced sodium excretion and weight gain.2,3 This traditional interpretation was challenged by results from a sex-specific analysis of BP trajectories over the adult life course based on four longitudinal community-based cohorts from the US.4 In this analysis, Ji et al. demonstrated that women have a steeper increase in BP that started in the third decade of life and continued throughout the life course.4 This sex difference was evident for systolic, diastolic and mean BP and for pulse pressure.4 These findings probably reflect differences in vascular biology between women and men. Anatomic and physiologic sex differences in the cardiovascular (CV) system are well described. Furthermore, socioeconomic, sociocultural and environmental factors, and sex differences in BP regulators including the sympathetic nervous system, the renin–angiotensin–aldosterone system, bradykinin, nitric oxide and brain natriuretic peptides are documented.3,5 Vascular inflammation, oxidative stress and organ damage may influence development of high BP in a sex-specific manner.2,6,7 Taken together, vascular compliance is physiologically lower in women, while arterial elasticity is higher during the reproductive age, reflecting vascular effects of progesterone and oestrogen.8,9 Weight gain and obesity are particularly relevant to the steeper increase in BP during the third decade of life in women because these factors influence CV organ function in a sex-specific manner.6,9 Furthermore, pre-eclampsia in pregnancy is associated with endothelial dysfunction and vascular inflammation, which is likely to persist beyond delivery.
Sex differences in Hypertension-associated Cardiovascular Organ Damage
Chronic hypertension causes structural and functional changes in the heart and arteries.10 Several studies have reported that hypertension-mediated organ damage in the heart and arteries is more prevalent in women than men, including left ventricular hypertrophy (LVH), left atrial (LA) dilatation and high arterial stiffness.11–15 Current guidelines recommend use of sex-specific partition values for optimal detection of hypertension-mediated organ damage, reflecting asymptomatic cardiovascular disease (CVD).10
LVH is the hallmark of hypertensive heart disease and a powerful prognostic marker in hypertension. In older patients in the LIFE echocardiography substudy, LVH was more prevalent in women both at study baseline (80% versus 70%) and after 4.8 years of systematic antihypertensive treatment (50% versus 34%).14 Of note, findings were similar in both obese and non-obese women, and persistent LVH was particularly associated with higher arterial stiffness. In middle-aged American Indians with hypertension participating in the Strong Heart Study, LVH was found in 36% of women and 23% of men.15 During the 4-year follow-up, a net increase in LVH prevalence was found despite antihypertensive treatment and good BP control. This lack of LVH reduction was attributed to persistent obesity and progressive reduction in renal function.15 Finally, among 12,329 middle-aged southern Italian subjects with treated hypertension, participating in the Campania Salute Network project, LVH was more prevalent in women than men (43% versus 32%).13 During follow-up of this Italian cohort, new-onset LVH was detected in 21% of participants, more commonly in women and in obese patients.16 Further analysis demonstrated that among subjects without LVH, women had a 35% lower risk than men of major CV events (hospitalisation for acute coronary syndromes [ACS], heart failure [HF] or AF or CV death) during a median of 4 years follow-up. However, when LVH was present, this sex difference disappeared and women and men had similar risk.13
A dilated LA is considered an early sign of hypertensive heart disease and has been associated with increased risk for AF, HF and ACS, irrespective of presence of LVH in hypertension.17 Although the LA is normally larger in men than women, several studies have documented that LA dilatation is more common in women in hypertension.11,18 In the LIFE study, 56% of older women and 38% of older men had LA dilatation, and antihypertensive treatment did not reduce LA size much in these subjects over a median of 4.8 years of follow-up.11 Furthermore, in middle-aged obese subjects without known CVD, participating in the FAT-associated Cardiovascular Dysfunction Study, LA dilatation was found in 77% of women and 62% of men, and was particularly associated with higher arterial stiffness.12
Arterial stiffness may be assessed by several prognostically validated methods including brachial or central pulse pressure, pulse pressure:stroke index ratio, augmentation index and carotid–femoral pulse wave velocity (cf-PWV).10 While pulse pressure, pulse pressure:stroke index ratio and augmentation index are all normally higher in women than men of a similar age, cf-PWV is higher in men.19,20 Increased arterial stiffness may antecede the onset of hypertension, in particular systolic hypertension, but is also a common type of organ damage in chronic hypertension, reflecting that age and BP are both important confounders. In the Framingham Heart Study, increased arterial stiffness assessed by cf-PWV was common, even in treated hypertension, and was found in 63% of controlled and 90% of uncontrolled subjects.19 CV risk increases in parallel with increasing cf-PWV or pulse pressure:stroke index ratio, independent of presence of hypertension.19,20
Sex Differences in the Association Between Mildly Elevated Blood Pressure with Cardiovascular Disease
The 2019 Global Burden of Disease study report confirmed that elevated systolic BP was the most important risk factor for CV death in women worldwide and the second most important risk factor in men, after smoking.21 Elevated systolic BP was also the most important risk factor for disability-adjusted life years both in women and men above the age of 50 years.
Emerging evidence suggests that elevated BP is particularly important for ACS risk in young and middle-aged women. While the overall incidence rates and mortality from ACS have decreased in western societies over the past decades, an increase in hospitalisation for ACS has been observed in young and middle-aged women in several countries.22,23 Although healthy young and middle-aged women have lower BP than men, women with ACS are more likely to have hypertension than men.22
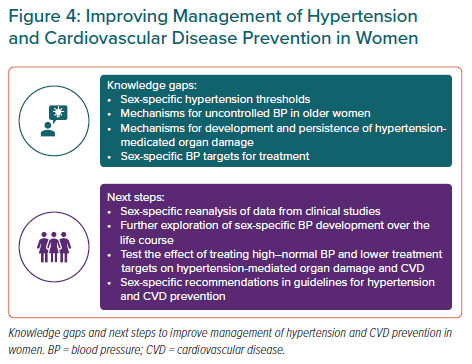
In a meta-analysis including 61 prospective studies of BP and mortality, a slightly stronger association between systolic BP and ischaemic heart disease mortality was found in women, particularly in the age group 40–50 years.24 At that time, the observation was not considered important. However, further publications have repeatedly documented a stronger association between BP and ACS in women than men. In the Norwegian Tromsø Study including 33,859 subjects aged 35–94 years, the associations between systolic and diastolic BP and MI were both stronger for women.25 In the UK Biobank study including 471,998 subjects free from CVD, with a mean age of 56 years, both BP 130–139/80–89 mmHg and BP ≥140/90 mmHg were associated with a 40% higher risk for MI in women compared with men during 8-year follow-up.26 Furthermore, in the Norwegian Hordaland Health Study, among 12,329 subjects initially aged 41 years followed for a median of 16 years, BP 130–139/80–89 mmHg was associated with a twofold higher risk for ACS during midlife in women, while no significant association was found in men (Figure 2).27 These studies all documented significant sex interaction tests.
In a meta-analysis of 16 population-based trials including 3,212,447 participants, mostly of Asian ethnicity, Han et al. found a stronger association between BP 130–139/80–89 mmHg and CVD in women than in men.28 In contrast, a pooled analysis of three prospective Chinese cohorts including 154,407 subjects aged 40–80 years found a comparable risk for CV death in women and men with BP 130–139/80–89 mmHg or hypertension, respectively.29
Hypertension is documented as the most important risk factor for HF in women, while previous MI is the most important risk factor for HF in men.6 While the lifetime risk of HF is similar in women and men, HF affects more women than men in absolute numbers.2 The Framingham Heart Study found HF with preserved ejection fraction (ejection fraction ≥50%) to be twice as common in women as in men.30 In a sex-specific pooled analysis of four community-based cohorts in the US, including 27,542 participants followed over a mean of 28 years, the risk for HF in women with systolic BP 110–119 mmHg was comparable to HF risk in men with systolic BP 120–129 mmHg. The association was more pronounced for women younger than 52 years, but the analysis did not differentiate between HF subtypes.31 In the same study, systolic BP was also a stronger risk factor for MI and stroke in women compared with men. Women with systolic BP 110–119 mmHg or 120–129 mmHg had comparable risk of MI and stroke to men with systolic BP 150–159 mmHg.31
Although women have lower age-adjusted incidence and prevalence of AF, the absolute number of women and men with AF is similar, probably reflecting the greater longevity of women.32 Among AF risk factors, hypertension, obesity and valvular heart disease are more prevalent in women, whereas coronary artery disease is more prevalent in men.33
A stronger association between systolic BP and risk for AF was reported in the Norwegian Tromsø Study.34 Among 16,046 40-year-old women and men followed for a median of 12.9 years, a persistently elevated systolic BP ≥140 mmHg during follow-up was associated with a twofold higher risk of AF in women, compared with a 50% increased risk in men. In further analysis expanding follow-up time to 17.6 years, increased systolic BP was associated with higher risk for both paroxysmal/persistent and permanent AF in women, but only for paroxysmal/persistent AF in men.35 Of note, increased AF risk was evident starting from systolic BP range 130–139 mmHg.
Should Sex-specific Thresholds for Hypertension be Introduced?
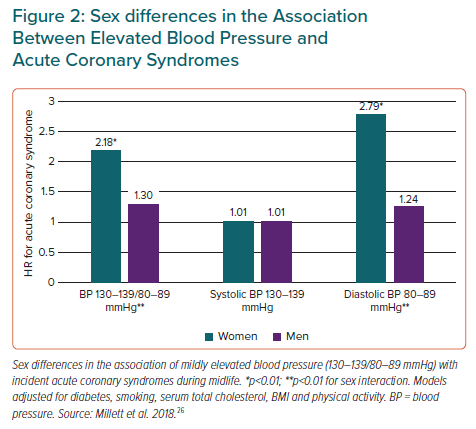
As presented above, publications exploring the sex-specific association of BP with risk for ACS, AF and HF have documented that CVD risk increases at a lower BP level in women than in men (Figure 3).25–28,31,34,35 The analyses have all been performed in community-based cohorts, and detailed data on antihypertensive drug use are limited. Furthermore, the findings reported in a research letter by Ji et al. need to be replicated in other data sets.31 However, the results are consistent and particularly strong for ACS, for which similar results have been documented from several cohorts.26,27,31,36 The more striking results in younger and middle-aged women suggest a potential for improved prevention of CVD, the most common cause of death and reduced disability-adjusted life years in women.21 The finding that CVD risk increases at a lower BP level in women may also offer an alternative explanation for the higher prevalence of hypertension-mediated organ damage in women than in men, which has traditionally been attributed to older age and sex differences in obesity and inflammatory disorders.
Few clinical studies in hypertension have reported results stratified by sex. In the DASH trial a pronounced antihypertensive effect of dietary sodium restriction was demonstrated in women.37 In the LIFE study, treatment effect was consistent in women and men, but more women in the losartan group were hospitalised for angina.38 The benefit of angiotensin-converting enzyme inhibitor treatment in reducing incident MI in the Second Australian National Blood Pressure Study Group was restricted to men only.39 Finally, similar benefits were demonstrated for women and men in the NORDIL study and the TOMHS.40,41 Taken together, results from sex-stratified and sex-specific analyses in clinical trials are limited, and reanalysis of data from older trials can be useful to rapidly increase knowledge and, at least in part, fill the knowledge gaps about optimal treatment of hypertension in women to prevent CVD.
Clinical studies testing the benefit of initiating antihypertensive drug treatment in women with BP 130–139/80–89 mmHg for reduction of CVD are lacking. In a systematic review and meta-analysis of 74 clinical trials, including 306,273 participants (39.9% women, mean age 64 years), Brunström et al. found null treatment effect of BP-lowering treatment in subjects with initial systolic BP <140 mmHg regarding all-cause mortality or CV morbidity or mortality in primary preventive trials.42 In contrast, in patients with known CHD, a reduction in incident stroke and HF was documented in patients with baseline systolic BP <140 mmHg. Unfortunately, no sex-specific results were presented in their publication.42
SPRINT, performed in 9,361 subjects with hypertension over the age of 50 years (36% women), documented that tighter BP control (systolic BP <120 mmHg) compared with standard BP control (systolic BP <140 mmHg) reduced major CV events (first occurrence of non-fatal MI, other ACS, stroke, HF hospitalisation, or CV death), but failed to demonstrate a statistically significant benefit for women, probably as a result of the lower proportion of included women.43 Still, SPRINT changed the US definition and management of hypertension for both sexes.
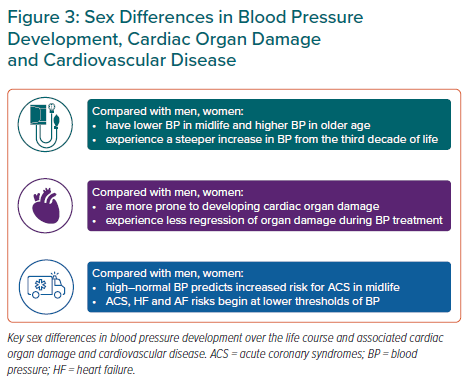
The limited information from clinical studies on benefit and treatment targets for women represents an important knowledge gap in effective CVD prevention in women (Figure 4). In particular, BP targets and the definition of hypertension in women need to be confirmed by sex-specific reanalysis of clinical data repositories and population-based cohorts complementing previous publications. Sex-related differences in pharmacokinetics and pharmacodynamics may impact optimal dosage, adverse effects and tolerability of antihypertensive drugs. Furthermore, based on the present criteria, uncontrolled hypertension remains more common in older women than their male counterparts.9 Whether this is related to sex differences in adherence, adverse effects or tolerability, or a result of higher prevalence of irreversible hypertension-mediated organ damage is not known. Thus, more knowledge on sex-specific underlying mechanisms is necessary to advance CVD prevention. This knowledge is necessary to optimise personalised prevention and management of hypertension and associated CVD in women.
Conclusion
It is well demonstrated that BP increases are steeper in women than men from the third decade of life, women are more prone to developing BP-associated cardiac organ damage and the BP-attributable risk for CVD starts at a lower level of BP in women than men (Figure 3). Based on current knowledge, we suggest that future guidelines on arterial hypertension and CVD prevention take into account the potential for implementing recommendations based on sex-specific association of high BP with CVD to improve risk assessment in young and middle-aged women with high normal BP. Whether different thresholds for sex-specific definitions of arterial hypertension would improve CVD risk detection should also be considered.